
Physics, 01.07.2021 15:30, kordejah348
A 3.10 mol sample of an ideal diatomic gas expands adiabatically from a volume of 0.1550 m3 to 0.742 m3 . Initially the pressure was 1.00 atm.(a) Determine the initial and final temperatures. initial Kfinal K(b) Determine the change in internal energy. J(c) Determine the heat lost by the gas. J(d) Determine the work done on the gas. J

Answers: 1
Other questions on the subject: Physics

Physics, 21.06.2019 17:20, issaaamiaaa15
An object thrown vertically upward from the surface of a celestial body at a velocity of 36 m/s reaches a height of sequalsminus0.9tsquaredplus36t meters in t seconds. a. determine the velocity v of the object after t seconds. b. when does the object reach its highest point? c. what is the height of the object at the highest point? d. when does the object strike the ground? e. with what velocity does the object strike the ground? f. on what intervals is the speed increasing?
Answers: 1


Physics, 22.06.2019 10:30, legendman27
You are given two vectors a⃗ =−3.00ι^ 5.00j^ and b⃗ =5.00ι^ 2.00j^. let the counterclockwise angles be positive.
Answers: 3

Physics, 22.06.2019 13:00, mckadams02
The magnitude of the amount of energy released by burning a fuel source, measured in energy per unit mass, is called its fuel value. note that the fuel value is the negative of the isobaric specific heat of combustion for the fuel. if all the energy obtained from burning 1.23 pounds of butane with a fuel value of 10.85 kcal/g is used to heat 128.0 kg of water at an initial temperature of 18.3 °c, what is the final temperature? note that 1 lb = 453.6 g.
Answers: 3
Do you know the correct answer?
A 3.10 mol sample of an ideal diatomic gas expands adiabatically from a volume of 0.1550 m3 to 0.742...
Questions in other subjects:

Mathematics, 07.07.2019 00:10

Mathematics, 07.07.2019 00:10





Mathematics, 07.07.2019 00:10


Geography, 07.07.2019 00:10


 = 1 atm = 101325 pascal
= 1 atm = 101325 pascal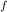 = ?
= ?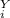 = P
= P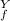
 V
V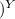
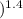
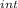 = nC
= nC ΔT
ΔT



