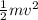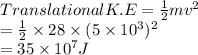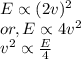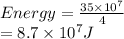The average speed of molecules of a 0.1 mole nitrogen gas in a container is 5103
m/s.
a/ Determine the total translational kinetic energy of the gas.
b/ Compute the energy as heat providing to the gas so that the average speed of its molecules increases to
double.

Answers: 1
Other questions on the subject: Physics

Physics, 21.06.2019 23:30, kennydenny4897
Ais not a compound machine. a. bolt b. shovel c. handcart (dolly) d. see-saw (teeter totter)
Answers: 2

Physics, 22.06.2019 08:00, Savageboyn
Gather reliable information to brent make his decision. to gather this information, use newspapers, call insurance companies or look at their web sites, and review consumer magazines and web sites. also, look at the manufacturer web site or for information about gas mileage. list the sources you use and take notes.
Answers: 1

Physics, 22.06.2019 18:30, breiajr
Aballoon is rising vertically upwards at a velocity of 10m/s. when it is at a height of 45m from the ground, a parachute bails out from it. after 3s he opens his parachute and decelerates ata a constant rate 5m/s. when. (a) what was the height of the parachutist above the ground when he opened his parachute? (b)how far is the parachutist from the balloon at t=3s? (c)with what velocity does the parachutist hit the ground? (d)after how long does the parachutist hit the ground after his exist from the balloon?
Answers: 3
Do you know the correct answer?
The average speed of molecules of a 0.1 mole nitrogen gas in a container is 5103
m/s.
a/ D...
a/ D...
Questions in other subjects:











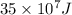 .
.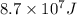 .
.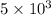 m/s
m/s