
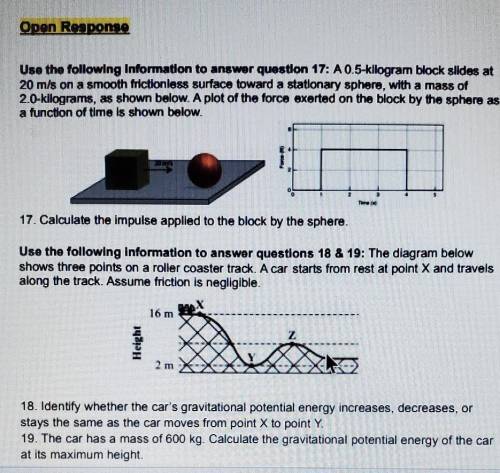
Use the following Infomadon to answer queston 174 A 0.5-klogram block alldos al 20 m/s on a smooth frictionless surface toward a stationary sphere, with a mass of 20-kllograms, as shown below. A plot of the forca exerted on the block by the spharga a function of tlma is shown below. 17. Calculate the Impulse applied to the block by the sphere. Use the following Information to answer questions 18 & 19: The dlagram below shows three points on a roller coaster track. A car starts from rest at polnt X and travels along the track. Assume friction is negligible. 16 m Helght HERE X 18. Identify whether the car's gravitational potential energy increases, decreases, or stays the same as the car moves from point X to point Y. 19. The car has a mass of 600 kg. Calculate the gravitational potential energy of the car at its maximum height.

Answers: 2
Other questions on the subject: Physics

Physics, 22.06.2019 11:30, joThompson
4. a 75.0 g piece of ag metal is heated to and dropped into 50.0 g of water at the final temperature of the mixture is what is the specific heat capacity of silver? 5. a 465 g chunk of iron is removed from petrucci, ralph h.. general chemistry (p. 290). pearson education. kindle edition.
Answers: 3

Physics, 22.06.2019 15:30, jjjjjj4999
Match each scenario to the form of energy it represents
Answers: 2


Physics, 23.06.2019 08:00, weeblordd
Use henry's law and the solubilities given below to calculate the total volume of nitrogen and oxygen gas that should bubble out of 1.7 l of water upon warming from 25 ˚c to 50 ˚c. assume that the water is initially saturated with nitrogen and oxygen gas at 25 ˚c and a total pressure of 1.0 atm. assume that the gas bubbles out at a temperature of 50 ˚c. the solubility of oxygen gas at 50 ˚c is 27.8 mg/l at an oxygen pressure of 1.00 atm. the solubility of nitrogen gas at 50 ˚c is 14.6 mg/l at a nitrogen pressure of 1.00 atm. assume that the air above the water contains an oxygen partial pressure of 0.21 atm and a nitrogen partial pressure of 0.78 atm.
Answers: 2
Do you know the correct answer?
Use the following Infomadon to answer queston 174 A 0.5-klogram block alldos al 20 m/s on a smooth f...
Questions in other subjects:



English, 30.08.2019 13:50

History, 30.08.2019 13:50

Social Studies, 30.08.2019 13:50

English, 30.08.2019 13:50


Arts, 30.08.2019 13:50

Mathematics, 30.08.2019 13:50






