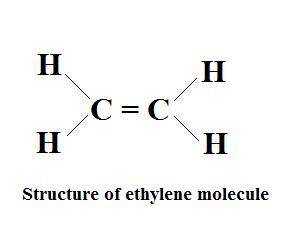
The compound ethylene (c2h4) is represented by this diagram. what statement best describes the arrangement of the atoms in an ethylene molecule? each carbon atom shares two electrons with one hydrogen atom and also shares two electrons with the other carbon atom. each carbon atom shares four electrons with one hydrogen atom and also shares four electrons with the other carbon atom. one electron is shared between each hydrogen atom and the carbon atom bonded to it, and two electrons are shared between the carbon atoms. two electrons are shared between each hydrogen atom and the carbon atom bonded to it, and four electrons are shared between the carbon atoms.

Answers: 1
Other questions on the subject: Physics

Physics, 22.06.2019 01:30, kayleetruman
The passing of heat through a material while the material itself stays in place. a. radiation b. conduction c. convection
Answers: 2

Physics, 22.06.2019 03:00, iicekingmann
Classify each possible hypothesis about a medicinal aloe vera plant as falsifiable or non-falsifiable. aloe vera gel is the best natural skin moisturizer. aloe vera gel can heal wounds by boosting cell renewal. aloe vera juice tastes better than carrot juice. drinking aloe juice can reduce the risk of lung cancer.
Answers: 1

Physics, 22.06.2019 19:30, bri1334
Listed below are the measured radiation absorption rates (in w/kg) corresponding to 11 cell phones. use the given data to construct a boxplot and identify the 5-number summary. 1.16 0.85 0.69 0.75 0.95 0.93 1.18 1.17 1.42 0.54 0.57 the 5-number summary is nothing, nothing, nothing, nothing, and nothing, all in w/kg. (use ascending order. type integers or decimals. do not round.)
Answers: 3

Physics, 22.06.2019 19:30, cecelia090
Another word for electromagnetic energy is a. heat b. force c. matter d. radiation
Answers: 1
Do you know the correct answer?
The compound ethylene (c2h4) is represented by this diagram. what statement best describes the arran...
Questions in other subjects:

Biology, 01.02.2020 17:43

Mathematics, 01.02.2020 17:43



History, 01.02.2020 17:43


Physics, 01.02.2020 17:43



History, 01.02.2020 17:43


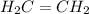 .
.