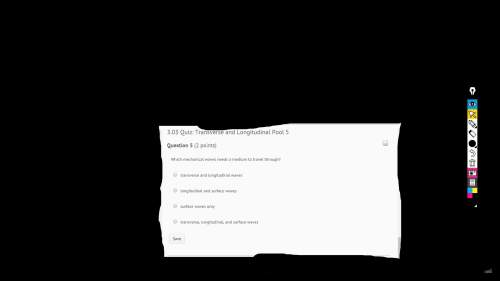
May i have some ? it would be really nice!
...

Answers: 2
Other questions on the subject: Physics

Physics, 21.06.2019 14:00, camballard3848
Two red blood cells each have a mass of 9.0 multiply. gif 10-14 kg and carry a negative charge spread uniformly over their surfaces. the repulsion arising from the excess charge prevents the cells from clumping together. one cell carries −2.50 pc of charge and the other −3.10 pc, and each cell can be modeled as a sphere 7.5 μm in diameter. (a) what speed would they need when very far away from each other to get close enough to just touch? assume that there is no viscous drag from any of the surrounding liquid. answer is: 321 m/s how do i find this (b) what is the maximum acceleration of the cells in part (a)? answer is: 1.38e+10 m/s2 how did they find this answer
Answers: 2

Physics, 22.06.2019 21:30, carleygalloway103
Calculate the minimum energy required to remove one proton from the nucleus 126c. this is called the proton-removal energy. (hint: find the difference between the mass of a 126c nucleus and the mass of a proton plus the mass of the nucleus formed when a proton is removed from 126c.) express your answer with the appropriate units. emin e m i n = nothing nothing request answer part b how does the proton-removal energy for 126c compare to the binding energy per nucleon for 126c, calculated using eb=(zmh+nmn−azm)c2?
Answers: 1

Physics, 22.06.2019 22:20, hailey5129
The starship enterprise is caught in a time warp and spock is forced to use the primitive techniques of the 20th century to determine the specific heat capacity of an unknown mineral. the 125-g sample was heated to 96.3°c and placed into a calorimeter containing 85.9 g of water at 20.0°c. the heat capacity of the calorimeter was 14.2 j/k. the final temperature in the calorimeter was 24.5°c. what is the specific heat capacity (in j/g°c) of the mineral? enter to 4 decimal places.
Answers: 1

Physics, 23.06.2019 01:00, lol23051
The amount of heat required to change liquid water to vapor at its boiling temperature is 2256 kj/kg. the amount of heat required to change liquid mercury to its vapor state at its boiling temperature is 295 kj/kg. one kg of each substance is currently at its boiling point. how will the amount of thermal energy required to change each substance from a liquid to a gas differ?
Answers: 3
Do you know the correct answer?
Questions in other subjects:


Mathematics, 31.08.2021 05:10

Mathematics, 31.08.2021 05:10

Mathematics, 31.08.2021 05:10

Biology, 31.08.2021 05:10

Mathematics, 31.08.2021 05:10

Mathematics, 31.08.2021 05:10

Social Studies, 31.08.2021 05:10

History, 31.08.2021 05:10

Mathematics, 31.08.2021 05:10