your


Answers: 3
Other questions on the subject: Physics


Physics, 21.06.2019 22:30, droidd133
Fft review: linspace, fs, fftshift, nfft 1. generate one second of a cosine of w,-10hz sampled at f, = 100hz and assign it to x. define a tt as your time axis 2. take 64 points fft. 3. as you remember, the dft (which the fft implements) computes n samples of s2t where k-0,1,2, n -1. plot the magnitude of this 64-points fft at range 0 to 63, what do you think of this graph? 4â·to get the x-axis into a hz-frequency form, plot this 64-points fft between-50 to 50 (the 100hz sampling rate) and have n-points between them. 5. according to your figure, what frequency is this cosine wave at? 6. remember that the fft is evaluating from 0 to 2ď€. we are used to viewing graphs from-ď€ to ď€. therefore, you need to shift your graph. 7. now according to your shifted graph. what frequency is this at? 8. note that the spikes have long drop-offs? try a 1024-point dft. note that the peak is closer to 10 and the drop-off is quicker. although, now sidelobes are an issue
Answers: 2

Physics, 22.06.2019 05:50, kamrulh278
Acylinder with a movable piston contains 11.7 moles of a monatomic ideal gas at a pressure of 1.32×10^5 pa. the gas is initially at a temperature of 300 k. an electric heater adds 43200 j of energy into the gas while the piston moves in such a way that the pressure remains constant. cp=20.79 j k^−1 mol^−1 for a monatomic ideal gas, and that the number of gas molecules is equal to avogadro's number (6.022×10^23) times the number of moles of the gas. (a) what is the temperature of the gas after the energy is added? (b) what is the change in volume of the gas? (c) how much work is done by the gas during this process?
Answers: 3

Physics, 22.06.2019 10:30, elijahjacksonrp6z2o7
The freezing and boiling point of a substance changes as the air pressure around it changes. for example, at a lower air pressure (higher altitude) it is easier for water molecules to escape from liquid into the air. in a high altitude city such as denver, colorado compared to a sea-level city such as houston, texas, water
Answers: 2
Do you know the correct answer?
As the skydiver falls to earth, she experiences positive acceleration due to
your
your
Questions in other subjects:

History, 10.03.2021 23:20


Geography, 10.03.2021 23:20

Social Studies, 10.03.2021 23:20



Mathematics, 10.03.2021 23:20

Computers and Technology, 10.03.2021 23:20

Mathematics, 10.03.2021 23:20

History, 10.03.2021 23:20


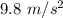 .
. 




