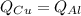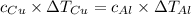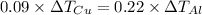Physics, 14.10.2019 09:30, krystalhurst97
Five-gram samples of copper and aluminum are at room temperature. both receive equal amounts of energy due to heat flow. the specific heat capacity of copper is 0.09 cal/g°c, and the specific heat capacity of aluminum is 0.22 cal/g°c. which of the following statements is true?
a. the temperature of each sample increases by the same amount.
b. the aluminum will get hotter than the copper.
c. the copper will get hotter than the aluminum.
d. the temperature of each sample decreases by the same amount

Answers: 1
Other questions on the subject: Physics

Physics, 22.06.2019 05:00, heavyhearttim
Asmall 21 kilogram canoe is floating downriver at a speed of 1 m/s. what is the canoe's kinetic energy?
Answers: 1

Physics, 22.06.2019 11:30, smilequi9653
Considering only the earth's rotation, determine how much later the asteroid would have had to arrive to put the explosion above helsinki at longitude 25˚ e? this would have obliterated the city.
Answers: 1

Physics, 22.06.2019 14:00, marieknight689
How much energy must a refrigerator absorb from 225 g of water so that the temperature of the water will drop from 35°c to 5°c
Answers: 3

Physics, 22.06.2019 15:30, salehabajwa101
What are the similarities & differences between a thermistor and a light dependent resistor in physics?
Answers: 1
Do you know the correct answer?
Five-gram samples of copper and aluminum are at room temperature. both receive equal amounts of ener...
Questions in other subjects:



Chemistry, 01.09.2019 13:10


Business, 01.09.2019 13:10

History, 01.09.2019 13:10

English, 01.09.2019 13:10

Computers and Technology, 01.09.2019 13:10



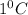
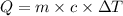
 = change in temperature
= change in temperature