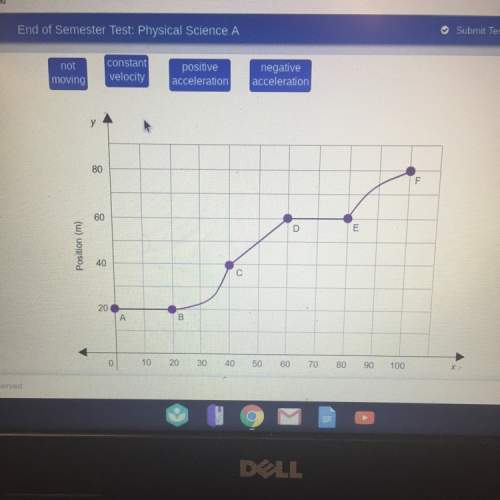
Physics, 15.04.2021 14:00, mimigg0621
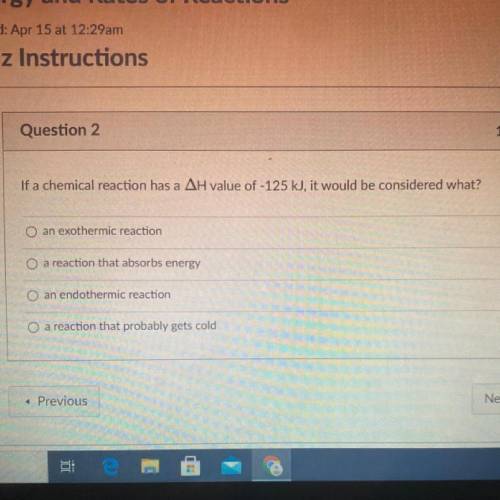
If a chemical reaction has a AH value of -125 kJ, it would be considered what?
A. an exothermic reaction
B. a reaction that absorbs energy
C. an endothermic reaction
D. A reaction that probably gets cold

Answers: 1
Other questions on the subject: Physics

Physics, 21.06.2019 22:30, leannamat2106
According to the tide table below what time of day will the highest tide occur?
Answers: 1

Physics, 22.06.2019 02:20, shadoris26
According to newton’s first law of motion, which force is expected to cause a body to accelerate?
Answers: 1

Physics, 22.06.2019 02:30, ntangpricha
Eddy whose mass is 55kg climbs up the 1.50 meter high stairs in 2s. calculate eddy’s power rating
Answers: 1

Physics, 22.06.2019 07:30, michaireid04
Identify the theory that can be used to explain each phenomenon. answers diffraction: wave theory interference: wave theory reflection: both particle and wave theories refraction: both particle and wave theories
Answers: 3
Do you know the correct answer?
If a chemical reaction has a AH value of -125 kJ, it would be considered what?
A. an exothermic rea...
Questions in other subjects:

Mathematics, 26.08.2019 01:30

Mathematics, 26.08.2019 01:30

French, 26.08.2019 01:30

English, 26.08.2019 01:30

English, 26.08.2019 01:30





Mathematics, 26.08.2019 01:30