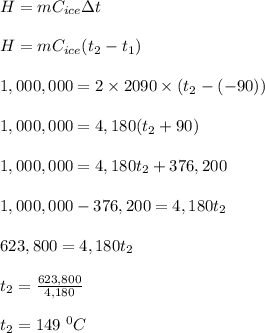The specific heat of water in its solid phase (ice) is 2090 J/(kg K), while in the liquid phase (water) its specific heat is 4190 J/(kg K). Water's latent heat of fusion is 333,000 J/kg. If you have a 2kg block of ice at -90°C and you add 1,000,000 J of heat, what is its new temperature?
a. 0°C
b. 14°C
c. 49°C
d. 149°C

Answers: 1
Other questions on the subject: Physics

Physics, 22.06.2019 11:30, swaggyd6603
Write down two additional questions about earth's magnetic field that will make your presentation more informative.
Answers: 1

Physics, 22.06.2019 17:00, WorkingButNotReally
If a negatively charged particle is placed at rest in an electric potential field that increases in the positive x-direction, what will the particle do? a. accelerate in the positive x-direction b. remain at rest c. accelerate in the negative x-direction
Answers: 3

Physics, 22.06.2019 17:30, Coolcatfurzy
Which basic property determines a colored light's placement on the spectrum of visible light? a. speed b. frequency c. amplitude d. velocity
Answers: 3
Do you know the correct answer?
The specific heat of water in its solid phase (ice) is 2090 J/(kg K), while in the liquid phase (wat...
Questions in other subjects:


Mathematics, 04.11.2021 21:00

Mathematics, 04.11.2021 21:00