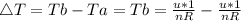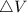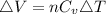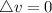Consider a system consisting of an ideal gas confined within a container, one wall of which is a movable piston. Energy can be added to the gas in the form of heat by applying a flame to the outside of the container. Conversely, energy can also be removed from the gas in the form of heat by immersing the container in ice water. Energy can be added to the system in the form of work by pushing the piston in, thereby compressing the gas. Conversely, if the gas pushes the piston out, thereby pushing some atmosphere aside, the internal energy of the gas is reduced by the amount of work done.

Answers: 3
Other questions on the subject: Physics

Physics, 21.06.2019 15:00, abbeygrace13
The optimistic view of human nature held by supporters of the humanistic perspective led to more positive views of human nature throughout the field of psychology. t or f?
Answers: 2

Physics, 22.06.2019 11:50, taylorannsalazar
Select all that applywhat are some basic resources a family is expected to provide for children? educationclothesspending
Answers: 2


Physics, 22.06.2019 15:30, tiffuuu
At 20∘c, the hole in an aluminum ring is 2.800 cm in diameter. you need to slip this ring over a steel shaft that has a room-temperature diameter of 2.804 cm . 1. to what common temperature should the ring and the shaft be heated so that the ring will just fit onto the shaft? coefficients of linear thermal expansion of steel and aluminum are 12×10−6 k−1 and23×10−6 k−1 respectively.
Answers: 1
Do you know the correct answer?
Consider a system consisting of an ideal gas confined within a container, one wall of which is a mov...
Questions in other subjects:

Mathematics, 23.10.2020 20:50


History, 23.10.2020 20:50


Mathematics, 23.10.2020 20:50

Mathematics, 23.10.2020 20:50

Mathematics, 23.10.2020 20:50

Mathematics, 23.10.2020 20:50



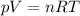
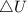 as the system of ideal gas goes from point A to point B on the graph recall u is proportional to T
as the system of ideal gas goes from point A to point B on the graph recall u is proportional to T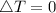
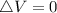
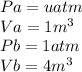
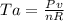
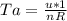
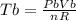
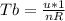
 is mathematically given as
is mathematically given as