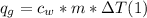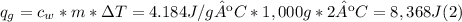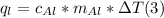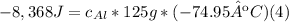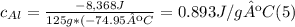Introduction: The specific heat capacity of a substance is the amount of energy needed to change the temperature of that substance by 1 °C. Specific heat capacity can be calculated using the following equation:
q = mc deltaT
In the equation q represents the amount of heat energy gained or lost in joules), m is the mass of the substance (in grams), c is the specific heat capacity of the substance (in J/g °C), and AT is the temperature change of the substance in °C).
Goal: Calculate the specific heat capacities of copper, granite, lead, and ice.
Solve: When you mix two substances, the heat gained by one substance is equal to the heat lost by the other substance. Suppose you place 125 g of aluminum in a calorimeter with 1,000 g of water. The water changes temperature by 2 °C and the aluminum changes temperature by -74.95 °C.
A. Water has a known specific heat capacity of 4.184 J/g °C. Use the specific heat equation to find out how much heat energy the water gained (q).
B. Assume that the heat energy gained by the water is equal to the heat energy lost by the aluminum. Use the specific heat equation to solve for the specific heat of aluminum. Aluminum's accepted specific heat value is 0.900 J/g °C. Use this value to check your work.

Answers: 1
Other questions on the subject: Physics

Physics, 21.06.2019 22:00, natethawsm
Na food processing facility, a spherical container of inner radius r1 5 40 cm, outer radius r2 5 41 cm, and ther- mal conductivity k 5 1.5 w/m · k is used to store hot water and to keep it at 100°c at all times. to accomplish this, the outer surface of the container is wrapped with a 800-w electric strip heater and then insulated. the temperature of the inner surface of the container is observed to be nearly 120°c at all times. as- suming 10 percent of the heat generated in the heater is lost through the insulation, (a) express the differential equation and the boundary conditions for steady one-dimensional heat conduction through the container, (b) obtain a relation for the variation of temperature in the container material by solving the differential equation, and (c) evaluate the outer surface tempera- ture of the container. also determine how much water at 100°c this tank can supply steadily if the cold water enters at 20°c.
Answers: 2

Physics, 22.06.2019 09:30, tsvijay121
Astone is dropped from a cliff and falls 9.44 meters. what is the speed of the stone when it reaches the ground? a. 13.6 m/sec b. 1.39 m/sec c. 185 m/sec d. 9.80 m/sec
Answers: 3

Physics, 22.06.2019 14:00, astigall4272
What is the force that opposes motion and works against the downward pull? a) friction b) gravity c) weight d) acceleration
Answers: 1

Physics, 22.06.2019 16:30, aliviadushane
He latent heat of vaporization for ethyl alcohol is 854 j/g. the amount of energy, rounded to the nearest whole number, needed to change 5.20 grams of ethyl alcohol from a liquid to a gas is
Answers: 2
Do you know the correct answer?
Introduction: The specific heat capacity of a substance is the amount of energy needed to change the...
Questions in other subjects:

Spanish, 01.08.2019 15:30



Social Studies, 01.08.2019 15:40


Mathematics, 01.08.2019 15:40


English, 01.08.2019 15:40

English, 01.08.2019 15:40

English, 01.08.2019 15:40