
Physics, 28.12.2020 14:00, zeesharpe05
When 1.34 g Zn(s) reacts with 60.0 mL of 0.750 M HCl(aq), 3.14 kJ of heat are produced. Determine the enthalpy change per mole of zinc reacting for the reaction: Zn(s) +2HCl (aq) → ZnCl2 (aq) +H2 (g)

Answers: 1
Other questions on the subject: Physics


Physics, 21.06.2019 23:10, itzlianne
A248-g piece of copper is dropped into 390 ml of water at 22.6 °c. the final temperature of the water was measured as 39.9 °c. calculate the initial temperature of the piece of copper. assume that all heat transfer occurs between the copper and the water. remember, the density of water is 1.0 g/m
Answers: 1

Physics, 22.06.2019 05:10, delawdermia27
Which diagram correctly demonstrates the various forces acting on a ball moving horizontally with some speed?
Answers: 2
Do you know the correct answer?
When 1.34 g Zn(s) reacts with 60.0 mL of 0.750 M HCl(aq), 3.14 kJ of heat are produced. Determine th...
Questions in other subjects:




Mathematics, 15.08.2019 07:30

Spanish, 15.08.2019 07:30


Mathematics, 15.08.2019 07:30




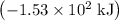 per mole
per mole 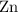 . (The negative value suggests the release of heat.) Assumption: the reaction was complete.
. (The negative value suggests the release of heat.) Assumption: the reaction was complete.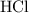 . Hence, either of the two species could be the limiting reactant. Calculating the number of moles of
. Hence, either of the two species could be the limiting reactant. Calculating the number of moles of 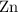 that was actually consumed requires finding the limiting reactant.
that was actually consumed requires finding the limiting reactant.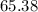 .
.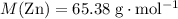 .
.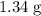 of
of 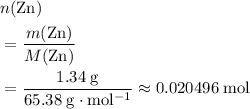 .
.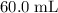 of
of 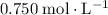 (
(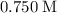 )
) 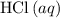 solution. Start by converting the unit of volume to liters (so as to match the unit of the concentration of this solution.)
solution. Start by converting the unit of volume to liters (so as to match the unit of the concentration of this solution.)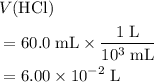 .
.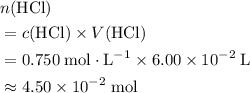 .
. .
.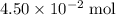 of
of 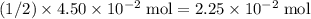
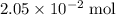 of
of 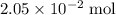 of
of 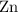 atoms would take part in this reaction.
atoms would take part in this reaction.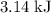 of heat through this reaction.
of heat through this reaction.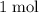 of
of 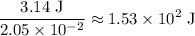 .
.



