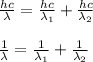A certain atom has only three energy levels. From lowest to highest energy, these levels are denoted n = 1, n = 2, and n = 3. When the atom transitions from the n = 3 level to the n = 2 level, it emits a photon of wavelength 800 nm. When the atom transitions from the n = 2 level to the n = 1 level, it emits a photon of wavelength 200 nm. What is the wavelength of the photon emitted when the atom transitions from the n = 3 level to the n = 1 level?A. 1000 nm
B. 600 nm
C. 500 nm
D. 160 nm

Answers: 1
Other questions on the subject: Physics



Physics, 23.06.2019 00:30, Tanya120
Which of the following statements are evidence that gases do not always behave ideally? check all that apply.? a. co2 gas becomes dry ice (solid co2) at 1 atm and –78.5 °c. b. when two gases are mixed, they follow dalton\'s law of partial pressures. c. at 4 k and 1 atm, helium is a liquid. d. it is impossible to compress a gas enough so that it takes up no volume.
Answers: 3

Physics, 23.06.2019 11:00, juniorvalencia4
Which temperature scale measures water boiling at 100 degrees? which temperature scale measures water boiling at 100 degrees?
Answers: 1
Do you know the correct answer?
A certain atom has only three energy levels. From lowest to highest energy, these levels are denoted...
Questions in other subjects:

SAT, 07.12.2021 20:40


Mathematics, 07.12.2021 20:40

Mathematics, 07.12.2021 20:40




Mathematics, 07.12.2021 20:40