
Physics, 06.11.2020 21:10, mackenzie112068
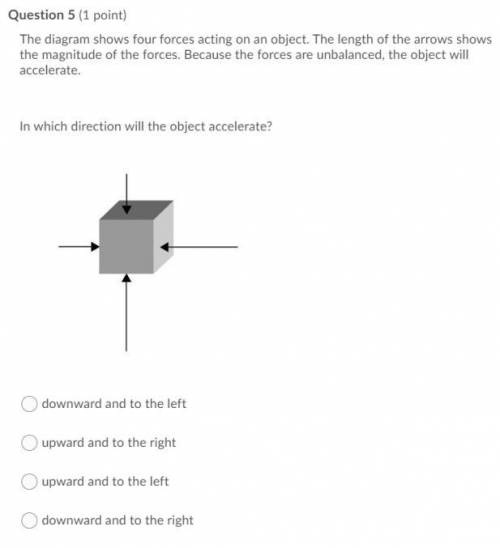
Please help lol
for the actual thing
will give brainliest





Answers: 1
Other questions on the subject: Physics

Physics, 22.06.2019 00:20, pooperjooper
Aparticle of mass m is projected with an initial velocity v0 in a direction making an angle α with the horizontal level ground as shown in the figure. the motion of the particle occurs under a uniform gravitational field g pointing downward. (a) write down the lagrangian of the system by using the cartesian coordinates (x, y). (b) is there any cyclic coordinate(s). if so, interpret it (them) physically. (c) find the euler-lagrange equations. find at least one constant of motion. (d) solve the differential equation in part (c) and obtain x and y coordinates of the projectile as a function of time. (e) construct the hamiltonian of the system, h, and write down the hamilton’s equations (canonical equations) of motion.
Answers: 2


Physics, 22.06.2019 01:10, caguil00
Asteam power plant operates on an ideal reheat rankine cycle between the pressure limits of 15 mpa and 10 kpa. the mass flow rate of steam through the cycle is 12 kg/s. steam enters both stages of the turbine at 500°c. if the moisture content of the steam at the exit of the low-pressure turbine is not to exceed 10 percent, determine (a) the pressure at which reheating takes place, (b) the total rate of heat input in the boiler, and (c) the thermal efficiency of the cycle. also, show the cycle on a t-s diagram with respect to the saturation lines. (2.15 mpa; 45.0 mw; 42.6%)
Answers: 3

Physics, 22.06.2019 18:50, jtsizemore
An insulated thermos contains 148 g of water at 72.7 ˚c. you put in a 11.7 g ice cube at 0.00 ˚c to form a system of ice + original water. the specific heat of liquid water is 4190 j/kg•k; and the heat of fusion of water is 333 kj/kg. what is the net entropy change of the system from then until the system reaches the final (equilibrium) temperature?
Answers: 2
Do you know the correct answer?
Please help lol
for the actual thing
will give brainliest
...
for the actual thing
will give brainliest
...
Questions in other subjects:

Mathematics, 13.10.2020 04:01


Chemistry, 13.10.2020 04:01

Mathematics, 13.10.2020 04:01

History, 13.10.2020 04:01

Mathematics, 13.10.2020 04:01

Mathematics, 13.10.2020 04:01



Mathematics, 13.10.2020 04:01






