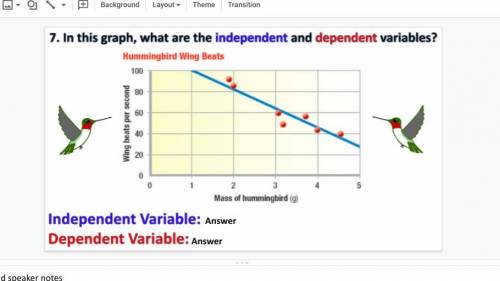
Free points if you answer this
...

Answers: 1
Other questions on the subject: Physics

Physics, 22.06.2019 02:10, lovelyheart5337
Explain the consequences of this addiction to the brain tissue
Answers: 1

Physics, 22.06.2019 11:00, coolfab9338
1.)the isotope cobalt-60 has a nuclear mass of 59.933820 u calculate the mass defect of cobalt-60 using the following information. mass of proton: 1.007825 u mass of neutron: 1.008665 u 1 u = 931.5 mev 2.)the isotope cobalt-60 has a nuclear mass of 59.933820 u calculate the binding energy of cobalt-60 using the following information. mass of proton: 1.007825 u mass of neutron: 1.008665 u 1 u = 931.5 mev 3.)the isotope cobalt-60 has a nuclear mass of 59.933820 u calculate the binding energy per nucleon of cobalt-60 using the following information. mass of proton: 1.007825 u mass of neutron: 1.008665 u 1 u = 931.5 mev
Answers: 3


Physics, 23.06.2019 09:30, triciajfive
According to rutherford's nuclear theory, most of the volume of an atom is , so the volume of a hydrogen atom cannot be mostly due to the proton. according to rutherford's nuclear theory, the core of an atom (nucleus) contains most of of an atom and is , so the majority of the mass of a fluorine atom cannot be due to its nine electrons. according to rutherford's nuclear theory, the number of negatively charged particles outside the nucleus the number of positively charged particles within the nucleus, so a nitrogen atom has 7 protons and 7 electrons, while a phosphorous atom cannot have 15 protons and 150 electrons. word bank: dense, empty space, more than, mass, particles, the same as, less than, positively charged, negatively charged
Answers: 2
Do you know the correct answer?
Questions in other subjects:

Computers and Technology, 25.11.2021 05:30




Social Studies, 25.11.2021 05:30





Social Studies, 25.11.2021 05:30