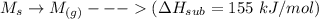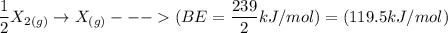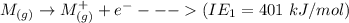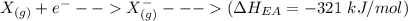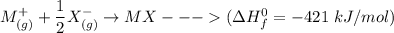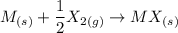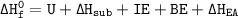Physics, 26.10.2020 17:00, 10242000cw
Consider an ionic compound, MXMX , composed of generic metal MM and generic, gaseous halogen XX . The enthalpy of formation of MXMX is ΔH∘f=−421ΔHf∘=−421 kJ/mol. The enthalpy of sublimation of MM is ΔHsub=155ΔHsub=155 kJ/mol. The ionization energy of MM is IE=401IE=401 kJ/mol. The electron affinity of XX is ΔHEA=−321ΔHEA=−321 kJ/mol. (Refer to the hint). The bond energy of X2X2 is BE=239BE=239 kJ/mol. Determine the lattice energy of MXMX .

Answers: 2
Other questions on the subject: Physics

Physics, 22.06.2019 03:30, Bryanguzman2004
Two polarizers are oriented at 24.0∘ to one another. light polarized at a 12.0-degree angle to each polarizer passes through both. what is the transmitted intensity (%)?
Answers: 2

Physics, 22.06.2019 14:00, marieknight689
How much energy must a refrigerator absorb from 225 g of water so that the temperature of the water will drop from 35°c to 5°c
Answers: 3

Physics, 22.06.2019 18:00, championroof
Acid precipitation chemically weathering a 5.0-kg limestone rock . which coukd be the result ?
Answers: 1
Do you know the correct answer?
Consider an ionic compound, MXMX , composed of generic metal MM and generic, gaseous halogen XX . Th...
Questions in other subjects:





English, 29.06.2019 02:40

Health, 29.06.2019 02:40

Mathematics, 29.06.2019 02:40

Mathematics, 29.06.2019 02:40


English, 29.06.2019 02:40


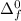 = - 421 kJ/mol
= - 421 kJ/mol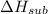 = 155 kJ/mol
= 155 kJ/mol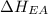 = -321 kJ/mol
= -321 kJ/mol