
Physics, 08.10.2020 01:01, ashtyndowdle
2. The visible region of the hydrogen spectrum results from relaxation of electrons from excited states to energy level 2 (n1). Use the Rydberg equation and your measured wavelengths to determine the energy transitions associated with each of your observed wavelengths for hydrogen. In other words, calculate the excited state energy level (n2) for each of your observed wavelengths for hydrogen. n has integer values; so, calculate it first with appropriate significant digits, then round it to an integer. Use the key to show your work for at least one calculation. Must show energy levels for each hydrogen wavelength.

Answers: 2
Other questions on the subject: Physics

Physics, 22.06.2019 11:30, joThompson
4. a 75.0 g piece of ag metal is heated to and dropped into 50.0 g of water at the final temperature of the mixture is what is the specific heat capacity of silver? 5. a 465 g chunk of iron is removed from petrucci, ralph h.. general chemistry (p. 290). pearson education. kindle edition.
Answers: 3

Physics, 22.06.2019 12:40, live4dramaoy0yf9
Find the equation for the plane through upper p 0 left parenthesis negative 4 comma negative 8 comma negative 5 right parenthesis perpendicular to the following line. xequalsnegative 4 minus t, yequalsnegative 8 plus 2 t, zequals3 t, minusinfinityless thantless thaninfinity
Answers: 2
Do you know the correct answer?
2. The visible region of the hydrogen spectrum results from relaxation of electrons from excited sta...
Questions in other subjects:



Computers and Technology, 29.07.2019 06:30



Mathematics, 29.07.2019 06:30


Biology, 29.07.2019 06:30



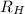 (1/2² - 1 / n²)
(1/2² - 1 / n²)




