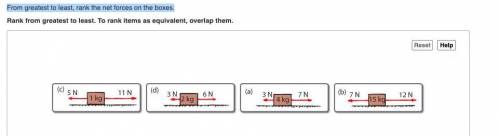
From greatest to least, rank the net forces on the boxes.
3n 4kg 7n
7n 15kg 12n
5n...


Answers: 3
Other questions on the subject: Physics

Physics, 21.06.2019 22:40, cookies1164
Ablock of mass m = 2.5 kg is attached to a spring with spring constant k = 710 n/m. it is initially at rest on an inclined plane that is at an angle of θ = 23° with respect to the horizontal, and the coefficient of kinetic friction between the block and the plane is μk = 0.19. in the initial position, where the spring is compressed by a distance of d = 0.16 m, the mass is at its lowest position and the spring is compressed the maximum amount. take the initial gravitational energy of the block as zero. a) what is the block's initial mechanical energy? b) if the spring pushes the block up the incline, what distance, l, in meters will the block travel before coming to rest? the spring remains attached to both the block and the fixed wall throughout its motion.
Answers: 3


Physics, 21.06.2019 23:30, 1031kylepoe03
Acoil formed by wrapping 80 turns of wire in the shape of a square is positioned in a magnetic field so that the normal to the plane of the coil makes an angle of 28.0° with the direction of the field. when the magnetic field is increased uniformly from 200 µt to 600 µt in 0.400 as, an emf of magnitude 80.0 mv is induced in the coil. what is the total length of the wire?
Answers: 3

Physics, 22.06.2019 05:50, kamrulh278
Acylinder with a movable piston contains 11.7 moles of a monatomic ideal gas at a pressure of 1.32×10^5 pa. the gas is initially at a temperature of 300 k. an electric heater adds 43200 j of energy into the gas while the piston moves in such a way that the pressure remains constant. cp=20.79 j k^−1 mol^−1 for a monatomic ideal gas, and that the number of gas molecules is equal to avogadro's number (6.022×10^23) times the number of moles of the gas. (a) what is the temperature of the gas after the energy is added? (b) what is the change in volume of the gas? (c) how much work is done by the gas during this process?
Answers: 3
Do you know the correct answer?
Questions in other subjects:


Biology, 03.04.2021 09:00

Mathematics, 03.04.2021 09:00


Biology, 03.04.2021 09:00

Physics, 03.04.2021 09:00

Mathematics, 03.04.2021 09:00