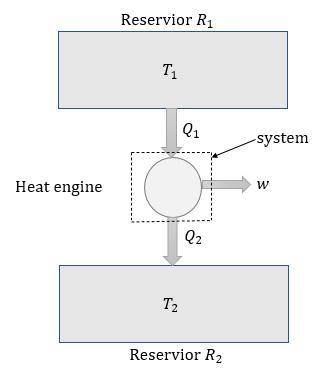
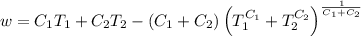A reversible heat engine, operating in a cycle, withdraws thermal energy from a high-temperature reservoir (the temperature of which consequently decreases), performs work w, and rejects thermal energy into a low-temperature reservoir (the temperature of which consequently increases). The two reservoirs are, initially, at the temperatures T1 and T2 and have constant heat capacities C1 and C2, respectively. Calculate the final temperature of the system and the maximum amount of work which can be obtained from the engine.

Answers: 2
Other questions on the subject: Physics

Physics, 22.06.2019 05:00, jocelynfray16
Which of the following is the result of the nuclear weak force? the instability of large nuclei the repelling force between positively charged protons the structure of the atom certain types of nuclear decay
Answers: 2

Physics, 22.06.2019 16:20, adiafloresp2dkbx
Specific heat refers to the amount of heat required to change 1 gram of a substance by degree(s) celsius
Answers: 1


Physics, 23.06.2019 02:50, neshaj
Adielectric-filled parallel-plate capacitor has plate area a = 30.0 cm2 , plate separation d = 9.00 mm and dielectric constant k = 3.00. the capacitor is connected to a battery that creates a constant voltage v = 15.0 v . throughout the problem, use ϵ0 = 8.85x10-12 c2/n. m2 . find the energy u1 of the dielectric-filled capacitor.
Answers: 2
Do you know the correct answer?
A reversible heat engine, operating in a cycle, withdraws thermal energy from a high-temperature res...
Questions in other subjects:


Computers and Technology, 01.07.2019 20:10




History, 01.07.2019 20:10



Social Studies, 01.07.2019 20:10


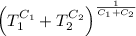
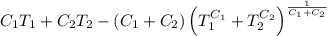 .
.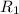 is the reservior having temperature
is the reservior having temperature 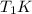 and heat capicity
and heat capicity 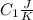 and
and 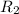 is the reservior having temperature
is the reservior having temperature 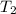 and heat capicity
and heat capicity 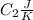 .
.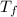 .
.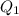 heat is extracted by the heat engine from the reservior
heat is extracted by the heat engine from the reservior 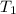 to
to 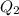 heat is rejected by the heat engine to the reservior
heat is rejected by the heat engine to the reservior 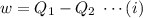
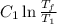 .
.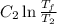 .
.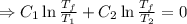
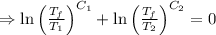
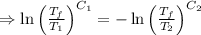
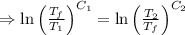
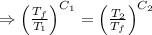 [taking anti-log both the sides]
[taking anti-log both the sides]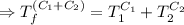
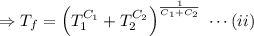
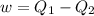
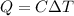
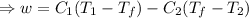
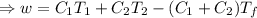
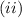 ,
,