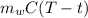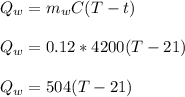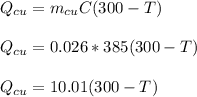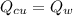Answers: 1
Other questions on the subject: Physics



Physics, 22.06.2019 11:00, coolfab9338
1.)the isotope cobalt-60 has a nuclear mass of 59.933820 u calculate the mass defect of cobalt-60 using the following information. mass of proton: 1.007825 u mass of neutron: 1.008665 u 1 u = 931.5 mev 2.)the isotope cobalt-60 has a nuclear mass of 59.933820 u calculate the binding energy of cobalt-60 using the following information. mass of proton: 1.007825 u mass of neutron: 1.008665 u 1 u = 931.5 mev 3.)the isotope cobalt-60 has a nuclear mass of 59.933820 u calculate the binding energy per nucleon of cobalt-60 using the following information. mass of proton: 1.007825 u mass of neutron: 1.008665 u 1 u = 931.5 mev
Answers: 3

Physics, 22.06.2019 14:40, babygirl091502
The experiment done in lab is repeated, using a ball that has unknown mass m. you plot your data in the form of f 2 versus m/l, with f in rev/s, m in kg, and l in m. your data falls close to a straight line that has slope 3.19 m/(kg · s2). use g = 9.80 m/s2 and calculate the mass m of the ball.
Answers: 1
Do you know the correct answer?
26.0 g of copper pellets are removed from a 300∘C oven and immediately dropped into 120 mL of water...
Questions in other subjects:

Mathematics, 19.10.2019 00:00

Mathematics, 19.10.2019 00:00

Advanced Placement (AP), 19.10.2019 00:00







Advanced Placement (AP), 19.10.2019 00:00


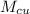 = 26 g = 0.026 kg
= 26 g = 0.026 kg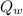 =
= 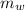 cΔθ =
cΔθ =