Which value would complete the last cell?
(1 point)
3.0
100.0
25.0...

Physics, 12.08.2020 08:01, jsndbdbsbsj
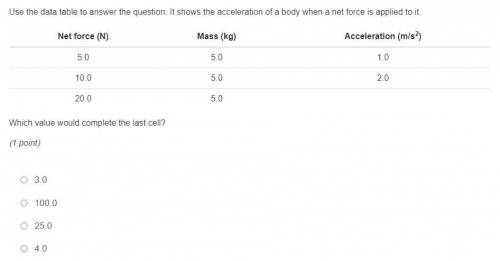
Which value would complete the last cell?
(1 point)
3.0
100.0
25.0
4.0

Answers: 1
Other questions on the subject: Physics

Physics, 22.06.2019 04:50, ineedhelpplz40
Two technicians are discussing a resistance measurement between the can-h and can-l wires. technician a says this measurement should be done with the ignition switch in the "run" position. technician b states that a measurement of 0 ohms indicates an open in the network. which technician is correct?
Answers: 1

Physics, 22.06.2019 05:40, thomasalmo2014
Unpolarized light of intensity i_0=750w/m^2 is incident upon two polarizers. after passing through both polarizers the intensity is i_2=280w/m^2. (a) what is the intensity of the light after it passes through the first polarizer in w/m^2? (b) write an equation for the angle between the polarizers in terms of the initial (i_0) and final (i_2) intensities. (c) find the angle between the polarizers in degrees.
Answers: 3

Physics, 22.06.2019 10:20, studyowl9192
Electromagnetic induction. a coil of wire contains n turns and has an electrical resistance r. the radius of each turn is a. initially, inside the coil there exists a uniform magnetic field of magnitude b0 parallel to the axis of the coil. the magnetic field is then reduced slowly. the current induced in the coil is i. how long does it take for the magnitude of the uniform field to drop to zero?
Answers: 1

Physics, 22.06.2019 10:50, milkshakegrande101
Asubject in a clinical research trial experiences a serious, unanticipated adverse drug experience. how should the investigator proceed, with respect to the irb, after the discovery of the adverse event occurrence? a. do not report the adverse drug experience to the irb since it is a common adverse experience. b. report the adverse drug experience to the irb only if there are several other occurrences. c. report the adverse drug experience as part of the continuing review report. d. report the adverse drug experience in a timely manner, in keeping with the irb's policies and procedures, using the forms or the mechanism provided by the irb.
Answers: 1
Do you know the correct answer?
Questions in other subjects:


English, 24.01.2020 00:31


Mathematics, 24.01.2020 00:31


Social Studies, 24.01.2020 00:31

Mathematics, 24.01.2020 00:31









