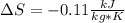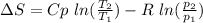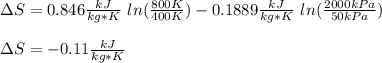Physics, 14.07.2020 17:01, oneicyahdaley10
Carbon dioxide initially at 50 kPa, 400 K, undergoes a process in a closed system until its pressure and temperature are 2 MPa and 800 K, respectively. Assuming an ideal gas behaviour, find the entropy change of the carbon dioxide by assuming that the specific heats are constant. For the gas, take Cp = 0.846 kJ/kg. K and R = 0.1889 kJ/kg. K

Answers: 3
Other questions on the subject: Physics

Physics, 22.06.2019 00:30, chayaharroch03
Imagine you work as a consultant, and your daily job involves listening to the constructive criticism of others. as part of your job, you must listen to opposing viewpoints to make important decisions. in this activity, you are a consulting dam engineer. you must make a decision about four different dams; should they be repaired, taken down, or left alone? you will listen to contrasting opinions about what should be done with the dams before you make the final decision. as the consulting dam engineer, what do you decide to do with this dam? explain your reasoning. by making your decision, did you support the opinions of the mayor and/or the dam safety official? why or why not? (site 1)
Answers: 1


Physics, 22.06.2019 10:00, myanniespencer39
Which accurately compares concave and convex lenses?
Answers: 2

Physics, 22.06.2019 16:20, brittanysanders
What is the mass of the water that is being heated? it requires 2,500 joules to raise a certain amount of water (c = 4.186 jig c) from 20.0°c to 60.0°c. o 159 o 40 g o 63 g o 80 g
Answers: 2
Do you know the correct answer?
Carbon dioxide initially at 50 kPa, 400 K, undergoes a process in a closed system until its pressure...
Questions in other subjects:



Mathematics, 17.10.2020 14:01


Mathematics, 17.10.2020 14:01

Chemistry, 17.10.2020 14:01

Advanced Placement (AP), 17.10.2020 14:01