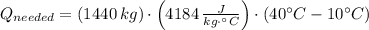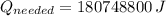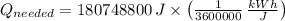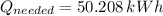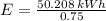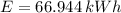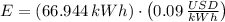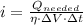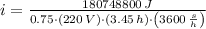(a) What is the cost of heating a hot tub containing 1440 kg of water from 10.0°C to 40.0°C, assuming 75.0% efficiency to take heat loss to surroundings into account? The cost of electricity is 9.00¢/(kW · h) and the specific heat for water is 4184 J/(kg · °C). $ 67 Incorrect: Your answer is incorrect. How much heat is needed to raise the temperature of m kg of a substance? How many joules are in 1 kWh? (b) What current was used by the 220 V AC electric heater, if this took 3.45 h? 88.2 Correct: Your answer is correct. A

Answers: 2
Other questions on the subject: Physics

Physics, 22.06.2019 08:00, hartzpeyton136
5g of ammonium nitrate was dissolved in 60g of water in an insulated container. the temperature at the start of the reaction was 23.0°c and at the end it was 19.0°c. calculate the energy absorbed by the reaction.
Answers: 3

Physics, 22.06.2019 16:20, jmackenzie7
How does a circuit breaker protect a refrigerator? a. when the current is too high, a metal strip in the fuse melts and opens the circuit. b. when the resistance is too high , a re-settable which opens a circuit c. when the current is too high , a re-settable switch opens the circuit d. when the resistance is too high a metal strip in the fuse melts and opens the circuit
Answers: 2

Physics, 23.06.2019 05:00, maljoh8249
Consider the phase change taking place in the picture. you could use all but one statement to describe what is happening here. that is: a) temperature is increasing. b) kinetic energy is increasing. c) the space between the particles remains the same. d) the attractive force between the particles is decreasing.
Answers: 1

Physics, 23.06.2019 10:40, puzzledprincess8037
4.at 0 ºc, some amount of energy is required to change 1 kg of water from a solid into a liquid. if you had a 2 kg piece of ice, what effect would this have on the amount of thermal energy required to change the water from a solid to a liquid? it would require more energy to change solid water into liquid water because there are more molecules in this larger piece of ice. it would still require the same amount of energy to change solid water into liquid water because the entire piece of ice would still gain the same amount of energy in each case. it would require energy to be removed from the 2 kg piece of ice. the larger piece of ice already has more total energy than the smaller piece of ice, so energy must be removed for the ice to become liquid. it would require less energy to change solid water into liquid water because the energy would spread through the ice more quickly and the ice already has a larger total amount of energy because it is larger than a 1 kg piece of ice.
Answers: 1
Do you know the correct answer?
(a) What is the cost of heating a hot tub containing 1440 kg of water from 10.0°C to 40.0°C, assumin...
Questions in other subjects:

Mathematics, 31.03.2020 02:38


Mathematics, 31.03.2020 02:38




Physics, 31.03.2020 02:38




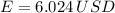 , For m kilograms, it is 4184m J., 3600000 joules, b)
, For m kilograms, it is 4184m J., 3600000 joules, b) 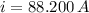
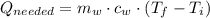
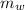 - Mass of water, measured in kilograms.
- Mass of water, measured in kilograms.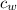 - Specific heat of water, measured in
- Specific heat of water, measured in 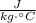 .
.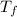 ,
, 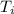 - Initial and final temperatures, measured in
- Initial and final temperatures, measured in 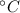 .
.