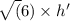Learning Goal:To understand and be able to use the rules for determining allowable orbital angular momentum states. Several numbers are necessary to describe the states available to an electron in the hydrogen atom. The principal quantum number ndetermines the energy of the electron. The orbital quantum number l determines the total angular momentum of the electron, and the magnetic quantum number mldetermines the component of the angular momentum parallel to a specific axis, usually the z axis. For a given principal quantum number n, the orbital quantum number can take integer values ranging from zero to n−1. For a given orbital quantum number l, the magnetic quantum number can take integer values from −l to l. A fourth number, the spin ms, is important for interactions with magnetic fields and counting states. The spin can be either +1/2 or −1/2, independent of the values of the other quantum numbers. The energy of an electron in hydrogen is related to the principal quantum number by En=(−13.60eV)/n2. The orbital angular momentum is related to the orbital quantum number by L=ℏl(l+1)−−−−−−√, and the orbital angular momentum in the z direction is related to the magnetic quantum number by Lz=mlℏ.
A. How many different values of I are possible for an electron with principal quantum number n = 5?
B. How many values of mi are possible for an electron with orbital quantum number I = 3? Express your answer as an integer.
C. The quantum state of a particle can be specified by giving a complete set of quantum numbers (n, l, m1, ms). How many different quantum states are possible if the principal quantum number is n = 3? To find the total number of allowed states, first write down the allowed orbital quantum numbers I, and then write down the number of allowed values of mi for each orbital quantum number. Sum these quantities, and then multiply by 2 to account for the two possible orientations of spin.
D. Is the state n = 3,1 = 3, m1 = -2, ms = 1/2 an allowable state? If not, why not?
a. Yes it is an allowable state.
b. No: The magnetic quantum number cannot be negative.
c. No: The magnetic quantum number must equal the orbital quantum number.
d. No: The orbital quantum number cannot equal the principal quantum number.
e. No: The magnetic quantum number must equal the principal quantum number.
E. What is the maximum angular momentum L max that an electron with principal quantum number n = 3 can have? Express your answer in units of h. (You don't need to enter the h, it is in the units field for you.)

Answers: 3
Other questions on the subject: Physics

Physics, 22.06.2019 03:30, Bryanguzman2004
Two polarizers are oriented at 24.0∘ to one another. light polarized at a 12.0-degree angle to each polarizer passes through both. what is the transmitted intensity (%)?
Answers: 2


Physics, 22.06.2019 10:30, laura52677
Agroup of students were investigating the force of gravity. they began by dropping a foam ball from a height of 3 meters into a bucket of sand. the ball hit the sand in 0.306 seconds. they dropped additional balls of approximately the same diameter, but of different masses. here is the data they collected. based on this experiment and the collected data, what would their conclusion be?
Answers: 1

Physics, 22.06.2019 16:00, russboys3
Agroup of monkeys is trying to cross the river in a rowboat. the maximum buoyant force on the rowboat is 2,000 n. the weight of the rowboat is 1,000 n. each monkey has a weight of 150 n. how many monkeys can safely cross in the rowboat at one time? explain your reasoning, including any calculations you used to find the answer.
Answers: 1
Do you know the correct answer?
Learning Goal:To understand and be able to use the rules for determining allowable orbital angular m...
Questions in other subjects:

Health, 08.01.2021 21:00

Biology, 08.01.2021 21:00





Physics, 08.01.2021 21:00

Mathematics, 08.01.2021 21:10

History, 08.01.2021 21:10

Health, 08.01.2021 21:10