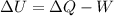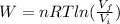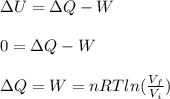An ideal monatomic gas at temperature T is held in a container. If the gas is compressed isothermally, that is at constant temperature, from a volume of Vi to Vf ,
a) What is the change in the (internal) energy of the gas?
b) How much work has been done on the gas?
c) Has heat been transferred into or out of the gas during the process? If so, how much?
d) Show that the 1st law of thermodynamics is satisfied.

Answers: 3
Other questions on the subject: Physics

Physics, 22.06.2019 03:00, iyanistacks50
Lymphocytes known as blastocysts make antibodies that fight infection. select the best answer from the choices provided t f
Answers: 2

Physics, 22.06.2019 06:00, pinkyglitter2696
What are atoms of the same element with varying number of neutrons
Answers: 3

Physics, 22.06.2019 11:20, puppylove899
Wave functions describe orbitals in a hydrogen atom. each function is characterized by 3 quantum numbers: n, l, and ml. if the value of n = 2: the quantum number l can have values from to . the total number of orbitals possible at the n = 2 energy level is .
Answers: 3
Do you know the correct answer?
An ideal monatomic gas at temperature T is held in a container. If the gas is compressed isothermall...
Questions in other subjects:









English, 26.08.2020 01:01