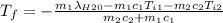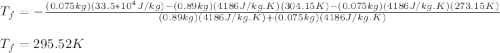Physics, 06.05.2020 04:44, Artemis3821
A thermos contains m1 = 0.89 kg of tea at T1 = 31° C. Ice (m2 = 0.075 kg, T2 = 0° C) is added to it. The heat capacity of both water and tea is c = 4186 J/(kg⋅K), and the latent heat of fusion for water is Lf = 33.5 × 104 J/kg. show answer No Attempt 50% Part (a) Input an expression for the final temperature after the ice has melted and the system has reached thermal equilibrium. Part (b) What is the final temperature in Kelvin?

Answers: 2
Other questions on the subject: Physics




Physics, 22.06.2019 21:00, thanitoast84
Ais a machine that makes work easier when a single force is applied. a simple machine b compound machine c endangered machine d complex machine
Answers: 2
Do you know the correct answer?
A thermos contains m1 = 0.89 kg of tea at T1 = 31° C. Ice (m2 = 0.075 kg, T2 = 0° C) is added to it....
Questions in other subjects:




Chemistry, 26.09.2019 20:00

Mathematics, 26.09.2019 20:00






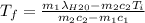
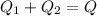
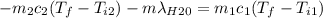 ( 1 )
( 1 )