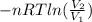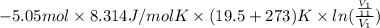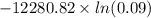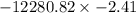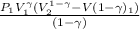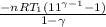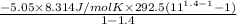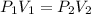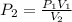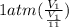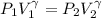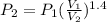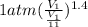Physics, 05.05.2020 16:10, shartman22
How much work is required to compress 5.05 mol of air at 19.5°C and 1.00 atm to one-eleventh of the original volume by an isothermal process? kJ (b) How much work is required to produce the same compression in an adiabatic process? kJ (c) What is the final pressure in part (a)? atm (d) What is the final pressure in part (b)? atm

Answers: 1
Other questions on the subject: Physics

Physics, 21.06.2019 22:00, coltonwsmith
In 1980, alfa romeo introduced the first system to smooth a rough idle caused by ignition and camshaft timing changes.
Answers: 1

Physics, 22.06.2019 03:50, am2garcia5
A30 kg weight lies on top of a massless piston of area a = 0.01 m2 the exterior air is at a (constant) p =1 atm and t = 27 c. the interior gas is 0.4 moles of (ideal) n2 and it has initial temperature 27.00 degrees c. 1. what is the initial pressure in the interior? a. 29.4 kpa b. 130.7 kpa c. 101.3 kpa the next three questions concern what happens when an amount of heat q is slowly added to the interior, raising the piston by 1 mm and raising the interior temperature to 27.40 c
Answers: 3


Physics, 22.06.2019 15:30, haileyjones732
Two pans of a balance are 24.1 cm apart. the fulcrum of the balance has been shifted 1.33 cm away from the center by a dishonest shopkeeper. by what percentage is the true weight of the goods being marked up by the shopkeeper? assume the balance has negligible mass. answer in units of %.
Answers: 1
Do you know the correct answer?
How much work is required to compress 5.05 mol of air at 19.5°C and 1.00 atm to one-eleventh of the...
Questions in other subjects: