
Physics, 06.05.2020 00:25, montamonta0204
The value of specific heat for copper is 390 J/kg⋅C∘, for aluminun is 900 J/kg⋅C∘, and for water is 4186 J/kg⋅C∘.
What will be the equilibrium temperature when a 235 g block of copper at 255 ∘C is placed in a 135 g aluminum calorimeter cup containing 825 g of water at 16.0 ∘C? Express your answer using three significant figures.

Answers: 3
Other questions on the subject: Physics

Physics, 22.06.2019 08:20, jrjordans13ox06qs
At an oceanside nuclear power plant, seawater is used as part of the cooling system. this raises the temperature of the water that is discharged back into the ocean. the amount that the water temperature is raised has a uniform distribution over the interval from 10° to 25° c. what is the standard deviation of the temperature increase?
Answers: 1


Physics, 22.06.2019 10:00, lorelei7668
(a) calculate the number of electrons in a small, electrically neutral silver pin that has a mass of 10.0 g. silver has 47 electrons per atom, and its molar mass is 107.87 g/mol. (b) imagine adding electrons to the pin until the negative charge has the very large value 1.00 mc. how many electrons are added for every 109 electrons already present
Answers: 3

Physics, 22.06.2019 10:00, kaniyawilhite
If a stone with an original velocity of 0 is falling from a ledgeand takes 8 seconds to hoybthe ground whays the final velocity of the stone
Answers: 2
Do you know the correct answer?
The value of specific heat for copper is 390 J/kg⋅C∘, for aluminun is 900 J/kg⋅C∘, and for water is...
Questions in other subjects:


Mathematics, 25.04.2020 02:10

Mathematics, 25.04.2020 02:10


History, 25.04.2020 02:10





Mathematics, 25.04.2020 02:10


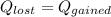
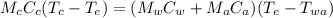
![0.235 \times 390\times(255-T_e)=([0.825\times4186]+[0.135\times900])(T_e -16)\\\\91.65(255-T_e)=(3453.45+121.5)(T_e -16)\\\\23370.75-91.65T_e=3574.95(T_e -16)\\\\23370.75-91.65T_e=3574.95T_e -57199.2\\\\3574.95T_e+91.65T_e=23370.75+57199.2\\\\3666.6T_e=80569.95\\\\T_e=\frac{80569.95}{3666.6} \\\\T_e=21.97](/tpl/images/0644/2367/dd73d.png)




