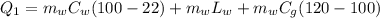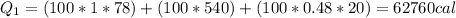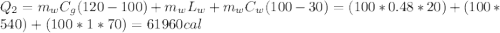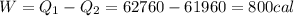Physics, 25.04.2020 05:08, haybaby312oxdjli
We are going to make an imaginary engine using water. We are going to heat 100 grams of water to 120 C from its initial temperature of 22 C. This would be the heat extracted from the hot reservoir. Then the steam will condense back into water and will be at a temperature of 30 C. This would be the exhaust expelled into the cold reservoir. You can assume the mass of the water and the mass of the steam is the same in this case. How much work can be done using this engine? The specific heat of water is 1 cal/(g C), the specific heat of steam is 0.48 cal/(g C),
and the latent heat of vaporization 540 cal/g.

Answers: 3
Other questions on the subject: Physics

Physics, 22.06.2019 10:00, jonlandis6
Need people build a dam to create a reservoir that supplies water a nearby city needs. describe two ways this action will likely affect the water cycle in the local environment. (5 points) worth 20 points
Answers: 1

Physics, 22.06.2019 11:00, MoogleCaliS
How do we measure or think of speed in the united states?
Answers: 1

Physics, 22.06.2019 16:40, odalysesquermon
The force needed to overcome static friction is usually less than that needed to overcome kinetic friction. true or false?
Answers: 1

Physics, 23.06.2019 00:00, alexandria3498
Three resistors are connected in series across a 15-v power supply. if the potential drops across resistors 1 and 2 are 4.1 volts and 3.1 volts, what is the exact potential drop (in volts) across resistor 3?
Answers: 2
Do you know the correct answer?
We are going to make an imaginary engine using water. We are going to heat 100 grams of water to 120...
Questions in other subjects:

Mathematics, 25.03.2020 11:24

English, 25.03.2020 11:24

Mathematics, 25.03.2020 11:24

English, 25.03.2020 11:25


English, 25.03.2020 11:26


Law, 25.03.2020 11:26

Biology, 25.03.2020 11:27