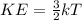Physics, 24.04.2020 08:55, salmirakayy4579
39. When you heat a flask of water, how are you changing the ordered kinetic energy of the
water molecules (if at all)? Likewise, how are you changing the random kinetic energy of the
molecules (if at all)?

Answers: 3
Other questions on the subject: Physics



Physics, 22.06.2019 21:30, itaheart101
What ia the energy of an object because of its potential and kinetic energies
Answers: 1

Physics, 22.06.2019 23:30, queenbroookk
Newton's law of cooling states that the rate of change in the temperature t(t) of a body is proportional to the difference between the temperature of the medium m(t) and the temperature of the body. that is, startfraction dt over dt endfraction equals upper k left bracket upper m left parenthesis t right parenthesis minus upper t left parenthesis t right parenthesis right bracket , where k is a constant. let kequals0.04 left parenthesis min right parenthesis superscript negative 1 and the temperature of the medium be constant, m(t) font size decreased by 3 equivalent font size decreased by 3 294 kelvins. if the body is initially at 369 kelvins, use euler's method with hequals0.1 min to approximate the temperature of the body after (a) 30 minutes and (b) 60 minutes.
Answers: 2
Do you know the correct answer?
39. When you heat a flask of water, how are you changing the ordered kinetic energy of the
wat...
wat...
Questions in other subjects:




English, 06.04.2020 19:32