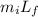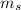Physics, 22.04.2020 03:55, camiserjai1832
What mass of steam at 100°C must be mixed with 490 g of ice at its melting point, in a thermally insulated container, to produce liquid water at 89.0°C? The specific heat of water is 4186 J/kg·K. The latent heat of fusion is 333 kJ/kg, and the latent heat of vaporization is 2256 kJ/kg.

Answers: 1
Other questions on the subject: Physics

Physics, 22.06.2019 05:30, trevorhenyan51
Suppose you have three polarizing filters, with the second at an angle of 42∘ to the first and the third at an angle of 90∘ to the first. by what perfect will the original intensity of unpolarized light be reduced to after passing through all three filters?
Answers: 2


Physics, 22.06.2019 10:30, zoebtharpe
An insulated 40 ft^3 rigid tank contains air at 50 psia and 120°f. a valve connected to the tank is now opened, and air is allowed to escape until the pressure inside drops to 25 psia. the air temperature during this process is kept constant by an electric resistance heater placed in the tank. determine the electrical work done during this process.
Answers: 2

Physics, 22.06.2019 19:50, nadiareese
Aleaky 10-kg bucket is lifted from the ground to a height of 15 m at a constant speed with a rope that weighs 0.7 kg/m. initially the bucket contains 45 kg of water, but the water leaks at a constant rate and finishes draining just as the bucket reaches the 15-m level. find the work done. (use 9.8 m/s2 for g.) show how to approximate the required work by a riemann sum. (let x be the height in meters above the ground. enter xi* as xi.) lim n → ∞ n (9.8)(45−3 xi) i = 1 δx express the work as an integral. 0 dx evaluate the integral. (round your answer to the nearest integer.) j
Answers: 3
Do you know the correct answer?
What mass of steam at 100°C must be mixed with 490 g of ice at its melting point, in a thermally ins...
Questions in other subjects:




History, 11.07.2019 19:00

History, 11.07.2019 19:00



Chemistry, 11.07.2019 19:00



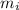 = 490 g = 0.49 kg
= 490 g = 0.49 kg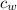 = 4186 J/kg.K = 4.186 kJ/kg.K
= 4186 J/kg.K = 4.186 kJ/kg.K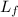 = 333 kJ/kg
= 333 kJ/kg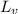 = 2256 kJ/kg
= 2256 kJ/kg