
Physics, 17.04.2020 12:32, zackarygonzalez1028
A/ Compute the quantity of heat released by 25.0 g of steam initially at 100.0oC, when it is cooled to 34.0°C and by 25.0 g of water initially at 100.0oC, when it is cooled to 34.0°C. b/ Suppose you put your hand into this steam or this water (it is not recommended!), in which case your hand will be burnt more severely?Explain. The heat of fusion of water is 2.256×106J/kg and the specific heat of water is 4.19×103J/kg. K

Answers: 1
Other questions on the subject: Physics




Physics, 23.06.2019 01:30, Dmoney5104
Match each type of lever with the correct diagram. 1. first-class 2. third-class 3. second-class
Answers: 3
Do you know the correct answer?
A/ Compute the quantity of heat released by 25.0 g of steam initially at 100.0oC, when it is cooled...
Questions in other subjects:



Biology, 17.07.2019 23:40






Chemistry, 17.07.2019 23:40

Social Studies, 17.07.2019 23:40


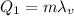
 is the latent heat of vaporization of water
is the latent heat of vaporization of water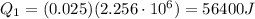

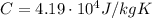 is the specific heat of water
is the specific heat of water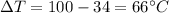 is the change in temperature
is the change in temperature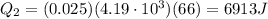
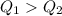 , we can say that your hand will burn more in the first case.
, we can say that your hand will burn more in the first case.



