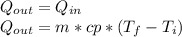reaches an equilibrium temperature of 31.1°C.

Physics, 17.04.2020 06:47, johnisawesome999
A jar of tea is placed in sunlight until it
reaches an equilibrium temperature of 31.1°C.
In an attempt to cool the liquid, which has a
mass of 177 g. 110 g of ice at 0.0°C is added.
At the time at which the temperature of the
tea is 29.1°C, find the mass of the remaining ice in the jar. The specific heat of water is 4186 J/kg.°C. Assume the specific heat capacity of the tea to be that of pure liquid water. Answer in units of g.

Answers: 2
Other questions on the subject: Physics

Physics, 21.06.2019 21:50, justintsmith6415
Which of the following is a homogenous mixture? o a. a toy box filled with toys o b. blood o c. trail mix o d. spaghetti and meatballs submit
Answers: 2

Physics, 22.06.2019 02:00, apalacios3503
Which safety measures should you follow during a thunderstorm? check all that apply. (a) avoid touching anything that conducts electricity. (b) avoid touching a person who has been struck by lightning. (c) go outside. (d) keep your computer turned off. (e) stay out of water. (f) stay inside.
Answers: 2

Physics, 22.06.2019 07:20, coolusername1314
The softest and hardest minerals are: softest hardest
Answers: 1

Physics, 22.06.2019 09:00, bendmads04
In a heat engine if 1000 j of heat enters the system the piston does 500 j of work, what is the final internal energy of the system if the initial energy was 2000 j? 1. write the equation 2.list out your known variables 3.plug the numbers into the equations 4.solve 5.write your solution statement that includes initial energy and final
Answers: 1
Do you know the correct answer?
A jar of tea is placed in sunlight until it
reaches an equilibrium temperature of 31.1°C.
reaches an equilibrium temperature of 31.1°C.
Questions in other subjects:


Business, 21.04.2020 19:02








Mathematics, 21.04.2020 19:02