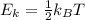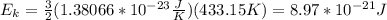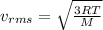A cylinder contains a mixture of helium and argon gas in equilibrium at 160°C.
(a) What...


Answers: 1
Other questions on the subject: Physics

Physics, 21.06.2019 14:40, shay68596
An implanted pacemaker supplies the heart with 72 pulses per minute, each pulse providing 6.0 v for 0.65 ms. the resistance of the heart muscle between the pacemaker’s electrodes is 550 ω. find (a) the current that flows during a pulse, (b) the energy delivered in one pulse, and (c) the average power supplied by the pacemaker.
Answers: 3


Physics, 22.06.2019 06:30, robertrkumar1
At very high pressures, gases become and will eventually a) more dense; become hotter b) more dense; change to a liquid or solid c) less dense; combust d) less dense; turn into a liquid
Answers: 2

Physics, 22.06.2019 10:30, jdkrisdaimcc11
What are two different ways you could find the value of a? explain these methods.
Answers: 2
Do you know the correct answer?
Questions in other subjects:

Biology, 26.08.2019 14:30


Chemistry, 26.08.2019 14:30



Biology, 26.08.2019 14:30

Health, 26.08.2019 14:30

Chemistry, 26.08.2019 14:30


Mathematics, 26.08.2019 14:30