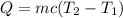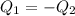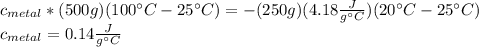Physics, 27.03.2020 17:07, camiee13dvivvj
En un experimento de calorimetría, 0.50 kg de un metal a 100°C se añaden a 0.50 kg de agua a 20°C en un vaso de calorímetro de aluminio, cuya masa es de 0.250 kg. A) Si un poco de agua salpica y sale del vaso al agregar el metal, el calor específico medido será 1) mayor, 2) igual o 3) menor que el valor calculado para el caso en que no se salpique agua. ¿Por qué? B) Si la temperatura final de la mezcla es de 25°C, y no se salpica agua, ¿qué calor específico tendrá el metal?

Answers: 1
Other questions on the subject: Physics

Physics, 22.06.2019 02:30, ParallelUniverse
Gunpowder residue is most likely to show up where on a shooters hands
Answers: 1

Physics, 22.06.2019 07:40, Alex9089435028
Astudent creates a model of a closed ecosystem by filling a glass tank half full with water, then adding 10 snails and two small aquatic plants. the next day, all the snails are dead. what is the most likely cause of their death?
Answers: 3

Physics, 22.06.2019 10:00, lorelei7668
(a) calculate the number of electrons in a small, electrically neutral silver pin that has a mass of 10.0 g. silver has 47 electrons per atom, and its molar mass is 107.87 g/mol. (b) imagine adding electrons to the pin until the negative charge has the very large value 1.00 mc. how many electrons are added for every 109 electrons already present
Answers: 3
Do you know the correct answer?
En un experimento de calorimetría, 0.50 kg de un metal a 100°C se añaden a 0.50 kg de agua a 20°C en...
Questions in other subjects:


English, 22.06.2019 13:00

English, 22.06.2019 13:00


English, 22.06.2019 13:00

Mathematics, 22.06.2019 13:00

Spanish, 22.06.2019 13:00