
Physics, 27.03.2020 05:16, Lovemimi4129
Using the following equation for the combustion of octane, calculate the heat associated combustion of 100.0 g of octane assuming complete combustion. The molar mass of octane is 114.33 g/mole. The molar mass of oxygen is 31.9988 g/mole.2C8H18+25O2→16CO2+18H2O;ΔH∘r xn = -11018 kJA. -11018 kJB. -4819 kJC. -602.3 kJD. -535.4 kJE. -385.5 kJ

Answers: 2
Other questions on the subject: Physics


Physics, 22.06.2019 16:20, punkieladie
What organization and environment is described above?
Answers: 1

Physics, 22.06.2019 17:00, bella51032
Explain what will happen if decomposers were apsent from a forest ecoysystem.
Answers: 1
Do you know the correct answer?
Using the following equation for the combustion of octane, calculate the heat associated combustion...
Questions in other subjects:

Mathematics, 18.01.2020 13:31

Biology, 18.01.2020 13:31




Chemistry, 18.01.2020 13:31

Mathematics, 18.01.2020 13:31


English, 18.01.2020 13:31


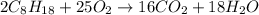 Δ
Δ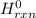 = -11018 kJ
= -11018 kJ



