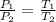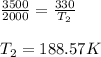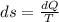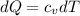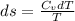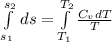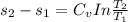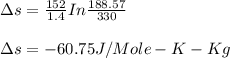Physics, 25.03.2020 23:41, starxx05235
Liquid chlorobenzene goes through a process where there is negligible heat flow and no work done on the surroundings. initially it is at 330 k and 3,500 kpa. at the end of the process the final pressure is 2,000kpa. estimate the temperature change and the entropy change of chlorobenzene.

Answers: 1
Other questions on the subject: Physics

Physics, 22.06.2019 18:00, pleasedontspamme
Consider an ideal gas at 27.0 degrees celsius and 1.00 atmosphere pressure. imagine the molecules to be uniformly spaced, with each molecule at the center of a small cube. what is the length l of an edge of each small cube if adjacent cubes touch but don't overlap?
Answers: 2

Physics, 22.06.2019 18:00, mariaaaaa69
Astudent pushes a 60-n block across the floor for a distance of 10 m. how much work was done to move the block
Answers: 1

Physics, 22.06.2019 22:00, shahrukhafridi2668
Which best term to describe a chemical reaction that absorbs energy from its surroundings? a. thermal conductor b. thermal insulator c. endothermic d. exothermic
Answers: 1

Physics, 22.06.2019 22:40, taniyahreggienae
Which of the following is a direct benefit of the use of assembly lines in production a. laws against theft fraud and coercion are needed to protect free choice. b. the government needs to plan efficient outcomes. c. competition among producers by itself cannot ensure a wide range of consumer choices. d. works pressure the government to guarantee minimum wages
Answers: 2
Do you know the correct answer?
Liquid chlorobenzene goes through a process where there is negligible heat flow and no work done on...
Questions in other subjects:

Mathematics, 13.01.2021 01:00


Mathematics, 13.01.2021 01:00