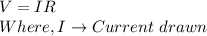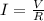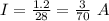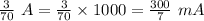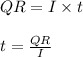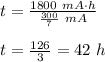The total charge a battery can supply is rated in mA*h, the product of the current (in mA) and the time (in h) that the battery can provide this current. A battery rated at 1000 mA*hr can supply a current of 1000 mA for 1.0 h, 500 mA current for 2.0 h, and so on. A typical AA rechargeable battery has a voltage of 1.2 V and a rating of 1800 mA*h.
A) For how long could this battery drive current through a long, thin wire of resistance 28 Ω ?

Answers: 1
Other questions on the subject: Physics

Physics, 22.06.2019 05:50, kamrulh278
Acylinder with a movable piston contains 11.7 moles of a monatomic ideal gas at a pressure of 1.32×10^5 pa. the gas is initially at a temperature of 300 k. an electric heater adds 43200 j of energy into the gas while the piston moves in such a way that the pressure remains constant. cp=20.79 j k^−1 mol^−1 for a monatomic ideal gas, and that the number of gas molecules is equal to avogadro's number (6.022×10^23) times the number of moles of the gas. (a) what is the temperature of the gas after the energy is added? (b) what is the change in volume of the gas? (c) how much work is done by the gas during this process?
Answers: 3

Physics, 22.06.2019 07:30, AnaiyaKirksey8
This is the substance(s) formed in a chemical reaction.
Answers: 1

Physics, 22.06.2019 11:20, leandrogarin37p2g5ds
Suppose a diode consists of a cylindrical cathode with a radius of 6.200×10^−2 cm , mounted coaxially within a cylindrical anode with a radius of 0.5580 cm . the potential difference between the anode and cathode is 260 v . an electron leaves the surface of the cathode with zero initial speed (v initial=0). find its speed vfinal when it strikes the anode.
Answers: 1

Physics, 22.06.2019 13:00, mckadams02
The magnitude of the amount of energy released by burning a fuel source, measured in energy per unit mass, is called its fuel value. note that the fuel value is the negative of the isobaric specific heat of combustion for the fuel. if all the energy obtained from burning 1.23 pounds of butane with a fuel value of 10.85 kcal/g is used to heat 128.0 kg of water at an initial temperature of 18.3 °c, what is the final temperature? note that 1 lb = 453.6 g.
Answers: 3
Do you know the correct answer?
The total charge a battery can supply is rated in mA*h, the product of the current (in mA) and the t...
Questions in other subjects:



Mathematics, 26.10.2020 17:30



Law, 26.10.2020 17:30

Business, 26.10.2020 17:30