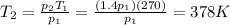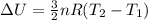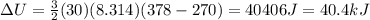A sealed tank contains 30 moles of an ideal gas at an initial temperature of 270 K. The pressure of the gas is increased until the final pressure equals 1.4 times the initial pressure. The heat capacity at constant pressure of the gas is 32.0 J(mol*K) What is the change in the internal energy of the gas? Let the ideal-gas constant R = 8.314 J/(mol • K).
130 kJ
77 kJ
-23 kJ
100 kJ
-50 kJ

Answers: 2
Other questions on the subject: Physics



Physics, 23.06.2019 02:30, morenodonaldo762
Can a molecule have bond dipoles but not have a molecular dipole?
Answers: 2
Do you know the correct answer?
A sealed tank contains 30 moles of an ideal gas at an initial temperature of 270 K. The pressure of...
Questions in other subjects:


Business, 17.06.2021 16:50

Mathematics, 17.06.2021 16:50

Mathematics, 17.06.2021 16:50







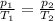
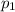 is the initial pressure of the gas
is the initial pressure of the gas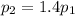 is the final pressure
is the final pressure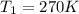 is the initial temperature
is the initial temperature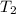 is the final temperature
is the final temperature