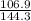You heat a 5.2 gram lead ball (specific heat capacity = 0.128 Joules/g-deg) to 183. ∘ C. You then drop the ball into 34.5 milliliters of water (density 1 g/ml; specific heat capacity = 4.184 J/g-deg ) at 22.4 ∘ C. What is the final temperature of the water when the lead and water reach thermal equilibrium?

Answers: 3
Other questions on the subject: Physics

Physics, 22.06.2019 09:50, ggcampos
Which statement correctly describes the current in a circuit that is made up of any two resistors connected in parallel with a battery? a. the current in the battery and in each resistor is the same b. the current the battery equals to the sum of the currents in the resistors c. the current in the battery is less than the current in either resistor d. the current in the battery equals to the product of the currents in the resistors
Answers: 1



Physics, 22.06.2019 15:30, jonathanmagana112002
Charge is distributed along the entire x-axis with uniform density λ. how much work does the electric field of this charge distribution do on an electron that moves along the y-axis from y = a to y = b? (use the following as necessary: a, b, ε0, λ, and q for the charge on an electron.)
Answers: 3
Do you know the correct answer?
You heat a 5.2 gram lead ball (specific heat capacity = 0.128 Joules/g-deg) to 183. ∘ C. You then dr...
Questions in other subjects:

Mathematics, 05.02.2021 18:10







Social Studies, 05.02.2021 18:10

Mathematics, 05.02.2021 18:10

Social Studies, 05.02.2021 18:10


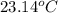
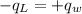 .............................1
.............................1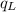 is the heat energy of the lead ball;
is the heat energy of the lead ball;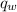 is the heat energy of water;
is the heat energy of water;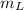 is the mass of the lead ball = 5.2 g
is the mass of the lead ball = 5.2 g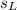 is the specific heat capacity of the lead ball = 0.128 Joules/g-deg
is the specific heat capacity of the lead ball = 0.128 Joules/g-deg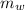 is the mass of water = volume x density = 34.5 ml x 1 g/ml = 34.5 g
is the mass of water = volume x density = 34.5 ml x 1 g/ml = 34.5 g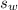 is the specific heat capacity of water = 4.184 J/g-deg
is the specific heat capacity of water = 4.184 J/g-deg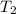 -22.4)
-22.4)