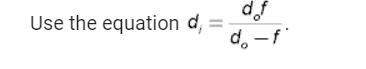The vapour pressure of pure liquid A at 300 K is 76.7 kPa and that of pure liquid B is 52.0 kPa. These two compounds form ideal liquid and gaseous mixtures. Consider the equilibrium composition of a mixture in which the mole fraction of A in the vapour is 0.350. Calculate the total pressure of the vapour and the composition of the liquid mixture.

Answers: 2
Other questions on the subject: Physics



Physics, 22.06.2019 18:50, rurbanok12
8.29 two streams containing pyridine and acetic acid at 25°c are mixed and fed into a heat exchanger. due to the heat-of-mixing effect, it is desired to reduce the temperature after mixing to 25°c using a stream of chilled ethylene glycol as indicated in the diagram. calculate the mass flow rate of ethylene glycol needed. the heat capacity of ethylene glycol at these conditions is approximately 2.8 kj/(kg k), and the enthalpy change of mixing (δmixh) is given below.
Answers: 3

Physics, 22.06.2019 19:30, alexmarche8637
Contains chemical energy. a. heat b. light c. natural gas
Answers: 2
Do you know the correct answer?
The vapour pressure of pure liquid A at 300 K is 76.7 kPa and that of pure liquid B is 52.0 kPa. The...
Questions in other subjects:


Mathematics, 17.08.2021 02:30


Mathematics, 17.08.2021 02:30

Mathematics, 17.08.2021 02:30

English, 17.08.2021 02:30

Mathematics, 17.08.2021 02:30

Chemistry, 17.08.2021 02:30