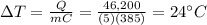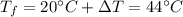A 5 kg block of copper (specific heat capacity = 385 J/kg°C) at 20°C is given
46,200 J of ener...

Physics, 10.03.2020 13:27, aprilreneeclaroxob0c
A 5 kg block of copper (specific heat capacity = 385 J/kg°C) at 20°C is given
46,200 J of energy. What will be its final temperature?

Answers: 3
Other questions on the subject: Physics

Physics, 21.06.2019 19:00, familyk0jj3
Write a question about how changing temperature affects the gas inside the sports ball
Answers: 1

Physics, 21.06.2019 23:30, mmaglaya1
Ablock of mass m = 2.5 kg is attached to a spring with spring constant k = 790 n/m. it is initially at rest on an inclined plane that is at an angle of 28 degrees with respect to the horizontal, and the coefficient of kinetic friction between the block and the plane is k = 0.14. in the initial position, where the spring is compressed by a distance of a = 0.18 m, the mass is at its lowest position and the spring is compressed the maximum amount. take the initial gravitational energy of the block as zero. b) if the spring pushes the block up the incline, what distance, l, in meters will the block travel before coming to rest? the spring remains attached to both the block and the fixed wall throughout its motion.
Answers: 1

Physics, 22.06.2019 08:30, 2021arabellacorsino
An object weigh 40n in air ,weigh 20n when submerged in water, and 30n when submerged in a liquid of unknown liquid density. what is the density of unknown of liquid?
Answers: 1

Physics, 22.06.2019 13:00, chie42
Which of the following correctly describes what happens when an atomic bomb explodes? small pieces of fissionable material are joined and form a body with a mass greater than the critical mass, the relative number of neutrons escaping decreases, and a chain reaction and explosion result. large pieces of fissionable matter are brought together quickly and form a body with a mass smaller than the critical mass, the relative number of escaping neutrons increases, and a chain reaction and explosion result.
Answers: 2
Do you know the correct answer?
Questions in other subjects:

Mathematics, 30.08.2021 05:30




Arts, 30.08.2021 05:30




Mathematics, 30.08.2021 05:30


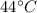
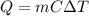
 is the increase in temperature
is the increase in temperature