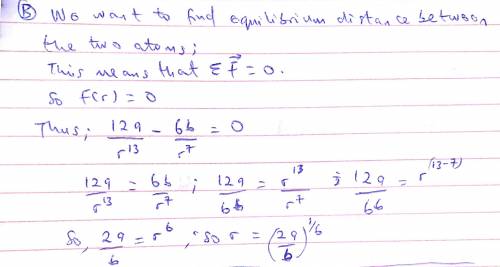Physics, 07.03.2020 06:12, Pumpkinputters
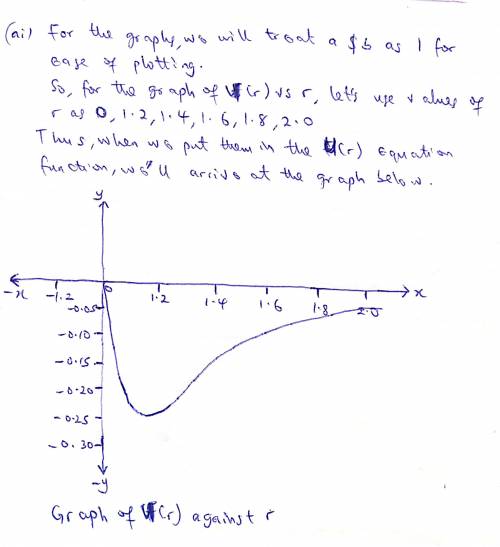
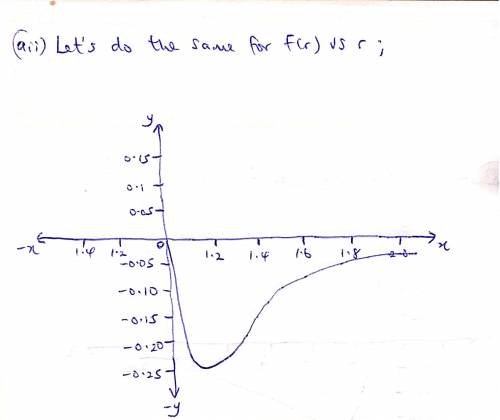
The potential energy of two atoms in a diatomic molecule is approximated by U(r)=(a/r12)−(b/r6), where r is the spacing between atoms and a and b are positive constants. (a) Find the force F(r) on one atom as a function of r. Draw two graphs: one of U(r) versus r and one of F(r) versus r. (b) Find the equilibrium distance between the two atoms. Is this equilibrium stable? (c) Suppose the distance between the two atoms is equal to the equilibrium distance found in part (b). What minimum energy must be added to the molecule to dissociate it—that is, to separate the two atoms to an infinite distance apart? This is called the dissociation energy of the molecule. (d) For the molecule CO, the equilibrium distance between the carbon and oxygen atoms is 1.13×10−10m and the dissociation energy is 1.54×10−18J per molecule. Find the values of the constants a and b.

Answers: 2
Other questions on the subject: Physics

Physics, 22.06.2019 09:30, 11needhelp11
(**i would really appreciate if this was answered soon** ) which pair of quantities includes one quantity that increases as the other decreases during simple harmonic motion?
Answers: 3

Physics, 22.06.2019 12:50, WritingStar1313
The combining of light nuclei is called blank. blank as in not actually blank. you know what im tryin to say.
Answers: 1

Physics, 22.06.2019 13:00, mandilynn22
Nacidified solution was electrolyzed using copper electrodes. a constant current of 1.18 a caused the anode to lose 0.584 g after 1.52 ✕ 103 s. given that the charge of an electron is 1.6022 ✕ 10−19 c, calculate avogadro's number. assume that copper is oxidized to cu2+ ions.
Answers: 1
Do you know the correct answer?
The potential energy of two atoms in a diatomic molecule is approximated by U(r)=(a/r12)−(b/r6), whe...
Questions in other subjects:


Chemistry, 06.10.2019 14:30




English, 06.10.2019 14:30

Biology, 06.10.2019 14:30