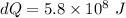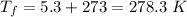Physics, 03.03.2020 00:51, curlyheadnikii
On a hot summer day, 3.50 ✕ 106 J of heat transfer into a parked car takes place, increasing its temperature from 36.5°C to 44.4°C. What is the increase in entropy (in J/K) of the car due to this heat transfer alone?
On a winter day, a certain house loses 5.80 ✕ 108 J of heat to the outside (about 550,000 Btu). What is the total change in entropy (in J/K) due to this heat transfer alone, assuming an average indoor temperature of 23.5°C and an average outdoor temperature of 5.30°C?

Answers: 3
Other questions on the subject: Physics

Physics, 22.06.2019 07:30, jaquezdaking4919
The slope of a velocity time graph over any interval of time gives the during that interval
Answers: 2

Physics, 22.06.2019 14:30, gabriellam20
A10nc charge sits at a point in space where the magnitude of the electric field is 1500 n/c. what will the magnitude of the field be if the 10 nc charge is replaced by a 20 nc charge? assume the system is big enough to consider the charges as small test charges.
Answers: 1

Physics, 22.06.2019 15:30, championroof
What is a costume plot? why is it important to a film or theater production?
Answers: 3
Do you know the correct answer?
On a hot summer day, 3.50 ✕ 106 J of heat transfer into a parked car takes place, increasing its tem...
Questions in other subjects:






Social Studies, 12.07.2019 22:00



Mathematics, 12.07.2019 22:00

History, 12.07.2019 22:00



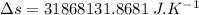
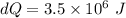
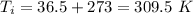

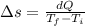
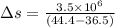 (heat is transferred into the system of car)
(heat is transferred into the system of car)