Physics, 26.02.2020 03:54, 1341220857
A sample of quartz, which has a specific heat capacity of 0.730 Jg1, is put into a calorimeter (see sketch at right) that contains 300.0 g of water. The quartz sample starts off a insulated 95.8 °C and the temperature of the water starts off at 15.0 °C, when the temperature of the water stops changing it's 17.7 °C. The pressure remains constant at 1 atm.
Calculate the mass of the quartz sample.

Answers: 3
Other questions on the subject: Physics



Physics, 22.06.2019 23:00, maddie6825
1700 j of energy is lost from 0.14 kg object , the temperature decreases from 50°c to 45°c what is the specific heat of this object, amd what is the material ?
Answers: 1

Physics, 23.06.2019 02:00, Ilovesnoopy69
Emergent properties of living systems are defined as properties that .
Answers: 3
Do you know the correct answer?
A sample of quartz, which has a specific heat capacity of 0.730 Jg1, is put into a calorimeter (see...
Questions in other subjects:

Chemistry, 25.11.2021 08:30




Business, 25.11.2021 08:30


Chemistry, 25.11.2021 08:30


Social Studies, 25.11.2021 08:40

Social Studies, 25.11.2021 08:40


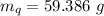
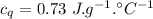
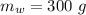
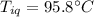
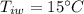
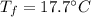
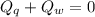
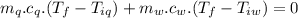
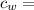 specific heat of water =
specific heat of water = 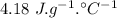 ,
,