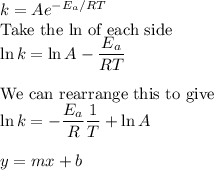Physics, 25.02.2020 23:32, deanazilyiah
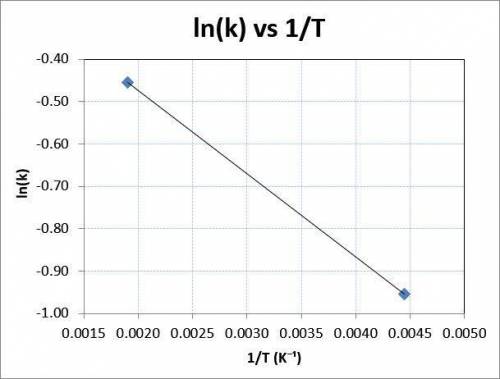
Consider the reaction data. A ⟶ products T ( K ) k ( s − 1 ) 225 0.385 525 0.635 What two points should be plotted to graphically determine the activation energy of this reaction? To avoid rounding errors, use at least three significant figures in all values. x 1 = y 1 = x 2 = y 2 = Determine the rise, run, and slope of the line formed by these points. rise: run: slope: What is the activation energy of this reaction? E a = J / mol

Answers: 1
Other questions on the subject: Physics



Physics, 22.06.2019 22:00, emmacox069
Ais not a mixture. colloid compound suspension solution
Answers: 1

Physics, 22.06.2019 23:30, queenbroookk
Newton's law of cooling states that the rate of change in the temperature t(t) of a body is proportional to the difference between the temperature of the medium m(t) and the temperature of the body. that is, startfraction dt over dt endfraction equals upper k left bracket upper m left parenthesis t right parenthesis minus upper t left parenthesis t right parenthesis right bracket , where k is a constant. let kequals0.04 left parenthesis min right parenthesis superscript negative 1 and the temperature of the medium be constant, m(t) font size decreased by 3 equivalent font size decreased by 3 294 kelvins. if the body is initially at 369 kelvins, use euler's method with hequals0.1 min to approximate the temperature of the body after (a) 30 minutes and (b) 60 minutes.
Answers: 2
Do you know the correct answer?
Consider the reaction data. A ⟶ products T ( K ) k ( s − 1 ) 225 0.385 525 0.635 What two points sho...
Questions in other subjects:


Biology, 29.11.2019 11:31

Chemistry, 29.11.2019 11:31




Mathematics, 29.11.2019 11:31


English, 29.11.2019 11:31

History, 29.11.2019 11:31