
Calculate the energy in the form of heat (in kJ) required to change 75.0 g of liquid water at 27.0 °C to ice at –20.0 °C. Assume that no energy in the form of heat is transferred to the environment. (Heat of fusion = 333 J/g; heat of vaporization = 2256 J/g; specific heat capacities: ice = 2.06 J/g·K, liquid water = 4.184 J/g·K)

Answers: 3
Other questions on the subject: Physics

Physics, 22.06.2019 03:00, nkidder
An electric current is flowing through the cord below. what will happen to this current if a magnet is brought near the cord? a. the electric current will stop flowing. b. it will exert a force on the voltage. c. the resistance of the wire will decrease. d. it will exert a force on the electric current.
Answers: 2


Physics, 22.06.2019 08:00, osmanysalvador9
Based on the concept of the wave-like nature of light, huygens' theory of light postulates that the more light was "bent" by a substance the slower it would move while traversing across that substance. a) deflection b) interference c) refraction d) resonance
Answers: 3

Do you know the correct answer?
Calculate the energy in the form of heat (in kJ) required to change 75.0 g of liquid water at 27.0 °...
Questions in other subjects:

Spanish, 03.02.2021 20:10

Mathematics, 03.02.2021 20:10

History, 03.02.2021 20:10

Biology, 03.02.2021 20:10

History, 03.02.2021 20:10

Mathematics, 03.02.2021 20:10

Mathematics, 03.02.2021 20:10

Mathematics, 03.02.2021 20:10

Biology, 03.02.2021 20:10

Mathematics, 03.02.2021 20:10


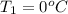
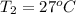
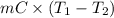
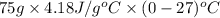
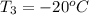 = (20 + 273) K = 293 K and specific heat of ice is 2.108 kJ/kg K.
= (20 + 273) K = 293 K and specific heat of ice is 2.108 kJ/kg K. 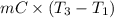
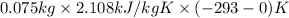
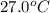 to ice at
to ice at 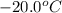 is -37.86 kJ.
is -37.86 kJ.



