

Answers: 1
Other questions on the subject: Physics

Physics, 21.06.2019 19:30, limelight11
Molten iron fills a mould, which has a volume of 200 cm cubed. calculate the volume when the iron cools and solidifies.
Answers: 3


Physics, 21.06.2019 22:00, moneybaggzay123
1. consider the case in which air fills air shocks on a truck trailer. the pressure in the shocks is 2 mpa. the temperature is 300 k. the diameter of the shock piston is 10 cm and the initial length of the cylindrical cavity containing the compressed air is 40 cm. a. the truck is gradually loaded over a period of a day in a static setting. the temperature is held constant for the atmosphere and thus for the gas shock. calculate the compressibility of the air in the shock for this condition when the truck is initially being loaded. b. if the shocks were loaded in a dynamic setting by driving over bumps, what would be the compressibility? state your assumption. c. what is the initial load on the shock if the shock is in an atmospheric 100 kpa? d. if the shock is compressed using the process described in part a, and the air shock compressed air cavity length decreases to 20 cm, what is the additional load applied to the shock?
Answers: 2
Do you know the correct answer?
Calculate the change in internal energy (ΔE) for a system that is giving off 25.0 kJ of heat and is...
Questions in other subjects:

Chemistry, 11.06.2020 16:57



Mathematics, 11.06.2020 16:57


Biology, 11.06.2020 16:57

Mathematics, 11.06.2020 16:57

Mathematics, 11.06.2020 16:57



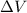 = (12 - 6) L = 6 L
= (12 - 6) L = 6 L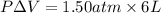
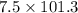
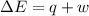
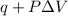
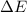 ) for a system is 24.240 kJ.
) for a system is 24.240 kJ.




