
Physics, 18.02.2020 01:05, electrofy456
A 15-ft3 tank contains oxygen initially at 14.7 psia and 80°F. A paddle wheel within the tank is rotated until the pressure inside rises to 20 psia. During the process 20 Btu of heat is lost to the surroundings. Determine the paddle-wheel work done. Neglect the energy stored in the paddle wheel. The gas constant and molar mass of oxygen are R = 0.3353 psia·ft3/lbm·R and M = 32 lbm/lbmol. The specific heat of oxygen at the average temperature of Tavg = (735 + 540)/2 = 638 R is cv ,avg= 0.160 Btu/lbm·R.

Answers: 3
Other questions on the subject: Physics

Physics, 21.06.2019 19:30, yairreyes01
Is it possible to use the energy from two atoms colliding to create a clean burning lasting fuel source with a large amount of force? is it also possible to harness that energy from the two atoms colliding to create a clean energy source that will last for years from just two atoms colliding. is there a possibility to control and store that energy for years but slowly let the energy out enough to power cities cheaper and faster.
Answers: 3


Physics, 22.06.2019 02:40, jordynsmith02
If the wheels lock when braking suddenly the vehicle: a: loses traction b: lose steering wheel ability c: gain speed slightly d: gain steering ability
Answers: 1

Do you know the correct answer?
A 15-ft3 tank contains oxygen initially at 14.7 psia and 80°F. A paddle wheel within the tank is rot...
Questions in other subjects:

Mathematics, 23.07.2021 19:10


Social Studies, 23.07.2021 19:10


Mathematics, 23.07.2021 19:10

Physics, 23.07.2021 19:10




History, 23.07.2021 19:10


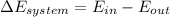
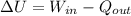
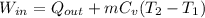
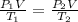
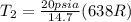
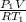
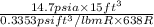
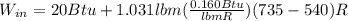
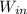 = 655.2 Btu
= 655.2 Btu



