Physics, 14.02.2020 05:25, Meap12345678910
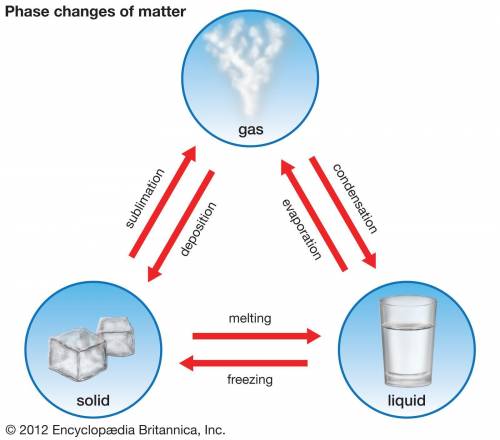
1. The change from a liquid state to a gaseous state deposition 2. The change from a gaseous state to a liquid state melting 3. The change from a solid state to a liquid state condensation 4. The change from a liquid state to a solid freezing 5. The change from a solid state to a gaseous state phase change 6. The change from a gaseous state to so solid boiling 7. A change of state sublimation

Answers: 3
Other questions on the subject: Physics

Physics, 22.06.2019 00:00, tsupreme
1134567 a jet with mass m = 1.1 ă— 105 kg jet accelerates down the runway for takeoff at 2 m/s2. 1) what is the net horizontal force on the airplane as it accelerates for takeoff? 2.2*10^5 n your submissions: 2.2*10^5 computed value: 220000submitted: saturday, january 26 at 2: 41 pm feedback: correct! 2) what is the net vertical force on the airplane as it accelerates for takeoff? 0 n your submissions: 0 computed value: 0submitted: saturday, january 26 at 2: 41 pm feedback: correct! 3) once off the ground, the plane climbs upward for 20 seconds. during this time, the vertical speed increases from zero to 21 m/s, while the horizontal speed increases from 80 m/s to 95 m/s. what is the net horizontal force on the airplane as it climbs upward? n 4) what is the net vertical force on the airplane as it climbs upward? n 5) after reaching cruising altitude, the plane levels off, keeping the horizontal speed constant, but smoothly reducing the vertical speed to zero, in 13 seconds. what is the net horizontal force on the airplane as it levels off? n 6) what is the net vertical force on the airplane as it levels off?
Answers: 1

Physics, 22.06.2019 04:00, scbmaster351
1. a student believes that colder water makes fish swim faster. he sets up an experiment using different temperatures of water and measures the speed of the fish. (chapter 1 – page 9) a. what is the independent variable? b. what is the dependent variable? c. list two constants the student should have for this experiment. 2. convert 0.375 mg to grams. show your work with units in order to receive credit. (chapter 1 – page 16) 3. a race car drives one lap around a race track that is 500 meters in length. (chapter 2 – pages 45-46) a. what is the driver’s displacement at the end of the lap? b. how is his displacement different from the distance traveled? 4. how far does a car travel in 90 seconds if it is traveling at a speed of 55 m/s? show the appropriate equation from your textbook and show your work with units in order to receive credit. (chapter 2 – pages 46-47) 5. two cars, both with a mass of 500 kg, are traveling down a road. the first car has a velocity of 65 m/s east and the second car has a velocity of 85 m/s west. (chapter 2 – pages 54-55) a. calculate the momentum of both cars showing the appropriate equation from your textbook and your work with units in order to receive credit. b. which car has the larger momentum? explain how you know. 6. an airplane traveling at 60 m/s comes to a stop in 10 seconds. calculate the airplane’s acceleration. show the appropriate formula and show your work with units in order to receive credit. (chapter 2 – pages 57-58) 7. an individual has a weight of 735 newtons. what is the individual’s mass? show the appropriate equation from your book and show your work with the units in order to receive credit. (chapter 3 – pages 78-79) 8. in terms of newton’s first law of motion, explain why it is important to wear a seatbelt while riding in a car. (chapter 3 – page 86) 9. if you kick a tennis ball with 50 n of force and then kick a soccer ball with 50 n of force, explain the difference in their motion according to newton’s second law. (chapter 3 – pages 81-82) 10. describe how the velocity and acceleration of a skydiver changes as she falls from the plane back to the ground. (chapter 3 – pages 88-89) 11. a child is swinging on swing. describe what happens to both the kinetic energy and potential energy of the child as she swings up and down. (chapter 4 – pages 123) 12. driving to work one morning, you get a flat tire. when using the car jack, you apply 120 n of force to the jack and the jack in turn applies 2000 n of force to lift the car up. what is the mechanical advantage of the jack? (chapter 4 – page 111) 13. a temperature of a 50 kg block increases by 15°c when 337,500 j of thermal energy are added to the block. (chapter 5 – pages 141-142) a. what is the specific heat of the object? show the appropriate equation from your book and show your work with units. b. what is the block made of? use the chart on page 141. c. is this block a good material for insulators or conductors? 14. explain why gases make better thermal insulators than solids or liquids. give one example from the textbook of a thermal insulator that can keep you warm on a cold day. (chapter 5 – pages 147) 15. several days after a snowfall, the roofs of some homes on your street have almost no snow on them, while the roofs on other homes are still snow covered. assuming they have all received the same amount of sunlight, give one reason for this observation related to thermal energy and insulation. 16. if you purchased a string of lights, how could you determine if the lights were wired in series or parallel? (chapter 6 – pages 185-186) 17. what happens to the current in a device if the resistance is decreased but the voltage stays the same? (chapter 6 – pages 181-182) 18. you measure the voltage difference of a circuit to be 15 v and the resistance to be 675 ω. what is the current in the circuit? show the appropriate equation from your book and show your work with units. (chapter 6 – page 182) 19. explain why a magnet from your refrigerator could not be used to lift something as heavy as a car. (chapter 7 – pages 202-203)
Answers: 3

Physics, 22.06.2019 07:10, PastyMexican24
1. how much energy is needed to raise the temperature of 40.0 g of argon from 25c to 40c? the specific heat capacity of argon is 0.520 j/(g·k) 2a. 23.0 ml of 0.100 m hcl (standard) are added from a buret to neutralize 50.0 ml of an unknown basic solution. 2b. if the oh- produced in the previous reaction came from ca(oh)2, then what is the molarity of the ca(oh)2? 3.calculate the new freezing-point of a solution when 60.5 grams of cacl2 solute is dissolved in 0.612 kg of water. 4. what is the maximum number of moles of alcl3 that can be produced from 5.0 mol al and 6.0 mol cl2? 5. a sample of oxygen gas has a volume of 150 ml when its pressure is 0.923 atm. if the pressure is increased to 0.987 atm and the temperature remains constant, what will the new volume be? 6. nitrogen gas in a closed container at a temperature of 100.0 oc and 3.0 atm is heated to 300 oc. what is the pressure of the gas at the higher temperature?
Answers: 3

Physics, 22.06.2019 10:00, issaaamiaaa15
Which is the correct symbol for an isotope of iodine with 53 protons and 78 neutrons?
Answers: 2
Do you know the correct answer?
1. The change from a liquid state to a gaseous state deposition 2. The change from a gaseous state t...
Questions in other subjects:

Arts, 01.07.2019 22:30



English, 01.07.2019 22:30

History, 01.07.2019 22:30

Mathematics, 01.07.2019 22:30



Chemistry, 01.07.2019 22:30